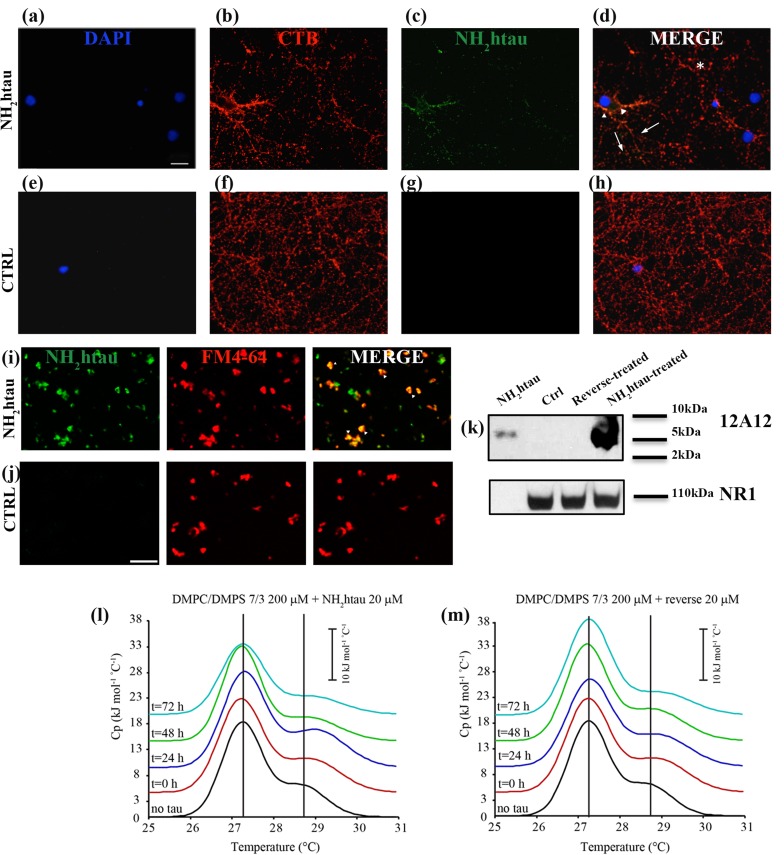
Figure 2. NH2htau is adsorbed by native neuronal plasma membranes under in vitro physiological conditions and deeply perturbs membrane-like lipid bilayers in a time-dependent manner.
a.-b.-c.-d.-e.-f.-g.-h. Fluorescence analysis (a-b-c-d) under non-permeabilizing conditions on hippocampal neurons (DIV15) in the presence or absence of the FITC-conjugated NH2htau. Live primary cultures were 30’exposed to FITC-conjugated NH2htau (1µM) (green channel) and then stained with TRITC-conjugated cholera toxin subunit b (red channel). Nuclei were stained with DAPI (blue channel). Merge image shows the composition of two fluorescence channels. Untreated cultures were used as negative control (e-f-g-h). Notice that NH2htau displays a diffuse, dot-like pattern distributed both in close proximity to and away from somatic compartment (arrowheads and arrows point to non-synaptic and synaptic areas, respectively). Structures which are positive for components of lipid rafts but not for NH2htau (asterisks) are also present. Scale bar:5 µm. i.-j. Fluorescence analysis of purified hippocampal synaptosomal fractions in the presence or absence of the FITC-conjugated NH2htau. Isolated synaptosomes were dual-labeled (i) by staining with FM4-64 (50µM for 1’+ 30mMKCl for 90sec) (red channel) and with FITC-conjugated NH2htau (1µM) for 10’(green channel). Untreated synaptosomes were used as negative control (j). Overlay image (yellow, arrowheads) indicates colocalization of NH2htau and FM 4-64 in numerous granular spots. Few ring-like morphological structures (usually 1–3 µm in diameter) resembling spherical synaptosomes are labeled by NH2htau but not by membrane-bound FM4-64 dye .Scale bar:10 µm. k. Western blotting analysis (n=2) of isolated membrane-containing fractions from primary hippocampal neurons (15 DIV) exposed for 1h to NH2htau and its reverse (5µM). Untreated cultures and synthetic NH2 26-44 human tau peptide were used as controls. Immunoblotting was performed with 12A12 monoclonal antibody (26-36 aa) and with NR1 subunit antibody, to check the preparation purity. Cropped representative WB are shown. l.-m. DSC thermograms of LUV DMPC/DMPS 7/3 freshly prepared (black curve), LUV DMPC/DMPS 7/3 + NH2htau or reverse 20 µM each t = 0 (red curve), LUV DMPC/DMPS 7/3 + NH2htau or reverse 20 µM t = 24h (blue curve), LUV DMPC/DMPS 7/3 + NH2htau or reverse 20 µM t = 48h (green curve), LUV DMPC/DMPS 7/3 + NH2htau or reverse 20 µM each t = 72h (cyan curve). Values from deconvolution analysis of the peak profiles are reported in Table 1.