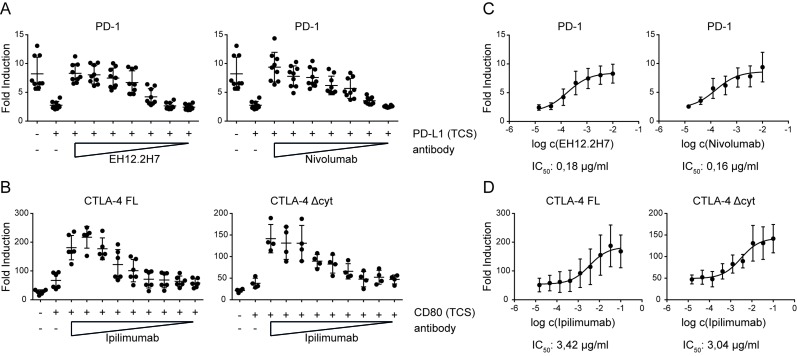
Figure 7. Evaluation of immune checkpoint inhibitors.
A. Control reporters (Ctrl) and PD-1 expressing reporters were stimulated with either TCS or TCS expressing PD-L1, and eGFP expression was measured via flow cytometry. PD-1 reporter cells were also stimulated with TCS-PD-L1 in presence of PD-1 antibodies (clone EH12.2H7 and Nivolumab, used at final concentrations of 10, 3.3, 1.1, 0.37, 0.12, 0.04, and 0.014 µg/ml). Results are shown from three independent experiments performed in triplicates. B. Control reporter (Ctrl) and reporters that express a full-length CTLA-4 (CTLA-4 FL) or a cytoplasmic deleted CTLA-4 (CTLA-4 Δcyt) were stimulated with either TCS or TCS expressing CD80, and eGFP expression was measured via flow cytometry. CTLA-4 FL and CTLA-4 Δcyt were also stimulated with TCS-CD80 in presence of Ipilimumab, used at 100, 33.3, 11.1, 3.7, 1.2, 0.4, 0.14, 0.04 and 0.015 µg/ml. Results are shown from six (FL) or four (Δcyt) independent experiments performed in triplicates. C. Half maximal inhibition concentrations (IC50) was calculated for the indicated blocking antibodies via a nonlinear regression curve followed by a one site competition using GraphPad Prism. D. Half maximal inhibition concentration (IC50) for Ipilimumab was calculated for CTLA-4 FL and CTLA-4 Δcyt via a nonlinear regression curve followed by a one site competition using GraphPad Prism.