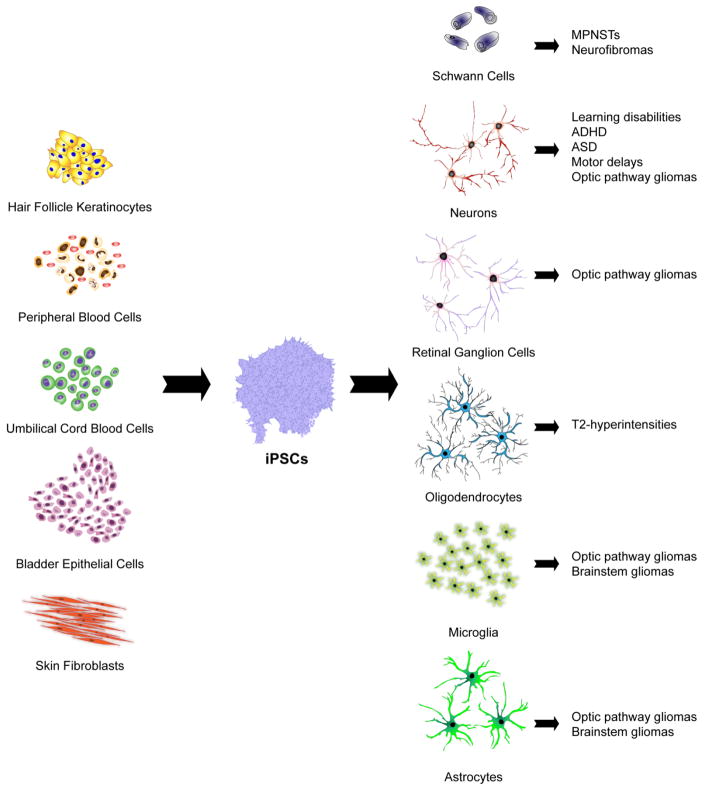
Fig. 2.
Patient-derived iPSCs can be generated by direct or indirect reprogramming of hair follicle keratinocytes, skin fibroblasts, urine-derived bladder epithelial cells, umbilical cord blood cells and peripheral blood cells. iPSCs can then be efficiently differentiated into the many distinct cell types that contribute to NF1 pathology using appropriate growth factors, cytokines, and extracellular matrix molecules. In this manner, iPSC-derived Schwann cells can be used to model malignant peripheral nerve sheath tumors (MPNSTs) and neurofibromas, while reprogrammed neurons can be employed to investigate the molecular etiologies of learning disabilities, autism spectrum disorder (ASD), attention-deficit-hyperactivity disorder (ADHD) and motor delays. Neurons, microglia and astrocytes comprise tumor microenvironments, and can enable modeling of optic pathway and brainstem gliomas, whereas reprogrammed retinal ganglion cells (RGCs) may be useful to evaluate the pathogenesis of optic glioma-associated RGC loss. Lastly, iPSC-derived oligodendrocytes can be employed to investigate the pathogenesis of the T2-weighted hyperintensities commonly found on magnetic resonance imaging (MRI) scans in young people with NF1.