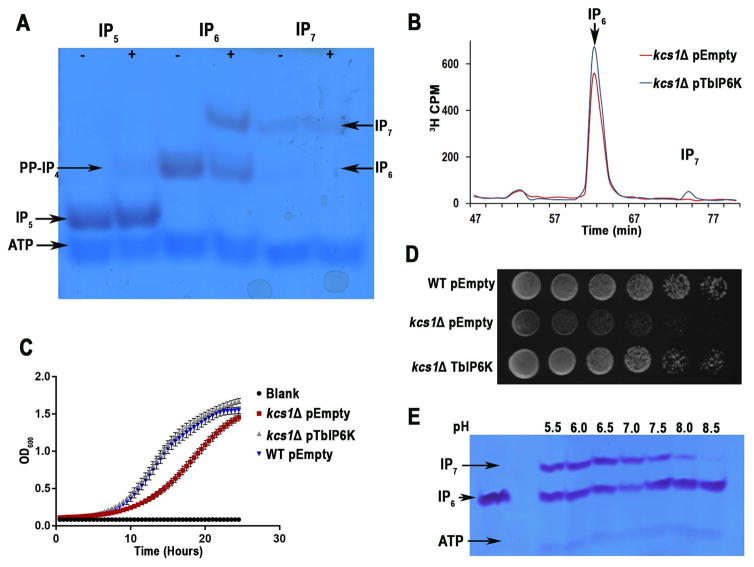
Fig. 4. TbIP6K activity and complementation of yeast mutants.
A. Kinase reactions performed with recombinant TbIP6K (2 μg) using the indicated substrates at 150 μM for 1 hour at 37°C. TbIP6K can phosphorylate I(1,3,4,5,6)P5 to PP-IP4 and IP6 to produce IP7 (5PP-IP5) but cannot phosphorylate IP7 to produce IP8. Other arrows show bands corresponding to ATP, and IP5.
B. HPLC analysis of soluble inositol phosphates of S. cerevisiae kcs1Δ mutants transformed with an empty vector (red) or a vector containing the entire open reading frame of TbIP6K (blue).
C. Growth of the same cells in liquid medium as estimated by measuring optical density at 660 nm. kcs1Δ mutants had reduced growth, which was restored by expression of TbIP6K. Mean ± s.d. for three independent experiments, each one with 6 duplicates.
D. WT, and kcs1Δ transformed with empty vector or kcs1Δ transformed with TbIP6K (serially diluted 10-fold, 106–10 cells/spot from left to right) were spotted on YPD plates and incubated at 30°C for 2 days.
E. Optimum pH for TbIP6K activity is under acidic conditions. We detected a higher activity at pH 6.0 and 6.5.
All results are representative of three or more independent experiments.