Abstract
Rank acquisition is a developmental milestone for young primates, but the processes by which primate yearlings attain social rank in the absence of the mother remain unclear. We studied 18 maternally reared yearling rhesus macaques (Macaca mulatta) that differed in their social and physical rearing environments. We found that early social experience and maternal rank, but not individual traits (weight, sex, age), predicted dominance acquisition in the new peer-only social group. Yearlings also used coalitions to reinforce the hierarchy, and social affiliation (play and grooming) was likely a product, rather than a determinant, of rank acquisition. Following relocation to a familiar environment, significant rank changes occurred indicating that familiarity with a physical environment was salient in rank acquisition. Our results add to the growing body of literature emphasizing the role of the social and physical environment on behavioral development, namely social asymmetries among peers.
Keywords: Rhesus macaque, dominance, coalitions, play, residency effect, peers, grooming
1. Introduction
From infancy, humans (Homo sapiens) are able to understand asymmetric relationships in social dominance (Gazes, Hampton, & Lourenco, 2015; Mascaro & Csibra, 2012; Pun, Birch, & Baron, 2016; 2017; Thomsen, Frankenhuis, Ingold-Smith, & Carey, 2011), with fully developed dominance relationships among peers by toddlerhood (Goldstein, Trancik, Bensadoun, Boyce, & Alder, 1999; Hawley & Little, 1999; Strayer & Strayer, 1976; Strayer & Trudel, 1984). This understanding suggests that asymmetric relationships are a fundamental part of human development. As Strayer & Trudel (1984, page 279) describe, “social dominance is developmentally the earliest stable dimension of peer group social organization”. So how do these relationships emerge?
The acquisition of dominance relationships has been a widely explored topic by both developmental psychologists and ethologists. Individual traits such as physical size and sex tend to predict dominance rank in social animals (humans, Hawley & Little, 1999; Pellegrini et al., 2007; Savin-Williams, 1977; reindeer, Rangifer tarandus: Holand et al., 2004; chimpanzees, Pan troglodytes: Pusey et al., 2005; rhesus macaques, Macaca mulatta: Angermeier, Phelps, Murray, & Reynolds, 1967), as this tends to reflect an individual’s “basic rank”, one based on isolated dyadic encounters (Holekamp & Smale, 1991; Kawai, 1958). In addition to physical traits, the degree of sociality displayed appears to play a fundamental role in how individuals can attain future ranks. Across a wide range of social animals, peers who engage in higher frequencies of social play tend to have higher dominance ranks (humans: Boulton, 1992; Hawley & Little, 1999; Smith & Boulton, 1990; yellow-bellied marmots, Marmota flaviventris: Blumstein, Chung, & Smith, 2013; Japanese macaques, Macaca fuscata: Norikoshi, 1974; chimpanzees: Paquette, 1994), probably because play behavior includes patterns typical of agonistic interactions (Pellis & Pellis, 1996), which helps refine skills for fighting. In social animals, however, individuals’ dominance rank might not depend exclusively on their own individual traits but might be contingent on the presence of other individuals who can influence the outcome of dyadic aggressive interactions through coalitionary interventions (humans: Harcourt & de Waal, 1992; Ross, Conant, Cheyne, & Alevizos, 1992; Russon, Waite, & Rochester, 1990; Strayer & Noel, 1986; chimpanzees: de Waal, 1982; Japanese macaques: Chapais, 1988a; 1988b; savannah baboons, Papio cynocephalus: Silk, Alberts, & Altmann, 2004; spotted hyenas, Crocuta crocuta: East et al., 2009). For example, coalitionary support from mothers and close kin can give rise to an interesting case of rank acquisition observed in many cercopithecine monkeys and in spotted hyenas, known as maternal rank inheritance (Kawai, 1958) where offspring attain adjacent dominance ranks to their mothers. However, it is unclear whether coalitionary interventions from mothers and matrilineal kin are needed to maintain their offspring’s rank (Altmann, 1980; Cheney, 1977; Gouzoules, 1975; Kawai, 1958). Cheney (1977) for instance showed that in chacma baboons (Papio ursinus) mothers and their families commonly intervene in support of their daughters, whereas in vervet monkeys (Cercopithecus aethiops), although daughters inherit the rank of their mothers, vervet mothers support their daughters during agonistic interactions in only 4% of the cases (Horrocks and Hunte, 1983). Moreover, in peer-only groups of rhesus macaques, infants reared in the absence of their mothers (i.e., nursery-reared) attain lower social ranks than mother-reared monkeys even after mother-reared infants are separated from their mothers (Bastian et al., 2003; Dettmer et al., 2016), suggesting that dominance rank can depend on early social experience, and normative social development is highly influenced by the mother’s presence early in development (Bastian et al., 2003). Additionally, if early social experience plays a pivotal role in rank acquisition, then infants living in multi-generational matrilines (MG) experience a larger and richer early social environment, than infants living in uni-generational groups (UG). While teasing apart the effect of social experience versus maternal and matrilineal support in free-ranging animals is challenging, this is possible to do in a laboratory setting, where different sets of social environments (MG vs UG) can be established, and infants or juveniles can be separated from their social group after a certain time period and raised together.
Finally, tenure in a social group also plays a role in rank development. For example, children that have been at a daycare longer also tend to be more dominant (Hawley & Little, 1999), and in rhesus monkeys, those introduced into a group earlier occupy higher ranks than those introduced later (Bernstein & Gordon, 1980; Snyder-Mackler et al., 2016), similar to group tenure in wild primates (chimpanzees: Foerster et al., 2016; but see Georgiev et al., 2016 for an unusual case in male rhesus macaques). The collective results of a variety of studies thus reveal that rank acquisition is a complicated process that likely depends on a number of individual and social factors, as well as the complexity of early life experiences. However, it remains unclear the relative importance of each of these factors in the process of rank development.
We had the unique opportunity to study rank acquisition in a newly established peer group of 8-mos old rhesus macaques, all of which were socially reared with their mothers and other peers. After the first 8-mos of life, they were removed and placed into a new peer-only social group, thus removing the possibility of the mother’s influence on peer interactions after group formation. Rhesus macaques are a good model to study the development of social rank because 1) they have genetic and physiological similarities to humans (Suomi, 1997), and 2) they have despotic linear hierarchies (Thierry, 2007) that are driven by strong nepotistic support (Bernstein & Ehardt, 1985). Importantly, however, the subjects differed in the social and physical environments in which they were reared: one group was reared in one of three large, floor-to ceiling cage-like enclosures with other peers, 10–12 same-aged adult females born in the same year, and one adult male. We termed this rearing “unigenerational” (UG) since only same-aged mothers and their infants were present. The other group was reared in a 5-acre, semi-naturalistic environment with multi-generational (i.e., grandmothers, great-grandmothers, aunts, cousins, and siblings) mixed-sex family groups (termed “multi-generational”, MG), providing us with the opportunity to examine potential rank differences based on the the complexity of the early social environment. We examined whether individual traits, early social experience, maternal rank, social behavior, and the physical environment influenced rank acquisition. We predicted that individual traits (weight, age, sex) would be unrelated to the acquisition of dominance rank (prediction 1) given the importance of maternal rank inheritance and other social mechanisms in rhesus macaques. Accordingly, we also predicted that MG subjects would outrank UG subjects, given the more complex social environment in MG groups (prediction 2). In addition, we hypothesized that maternal rank would continue to influence subsequent rank after peer group formation, given that all animals were mother-reared (prediction 3). This provided us with an opportunity to explore whether the first year of life was a critical period for infants to learn the necessary skills and relationships to acquire ranks similar to their mothers, even following permanent maternal separation. Additionally, we also examined social behavior, both in agonistic and affiliative contexts. We predicted that coalitions would be more prevalent in higher ranking monkeys to maintain their ranks (prediction 4a), and that coalitions would occur most frequently between individuals reared together (prediction 4b) due to kinship. Finally, given that play behavior can influence infants’ ability to acquire higher ranks, we predicted that individuals that played more would also have higher dominance ranks (prediction 5). Four months into the study, the social group was relocated to a physical environment that was identical to the early rearing environment of the UG subjects. We predicted that if familiarity with the physical environment was important for later dominance rank, the monkeys reared in this environment (the UG subjects) would be dominant after relocation (prediction 6). This relocation provided us with a unique opportunity to examine whether previous familiarity with a physical environment would contribute to rank acquisition.
2. Methods
2.1 Subjects and rearing
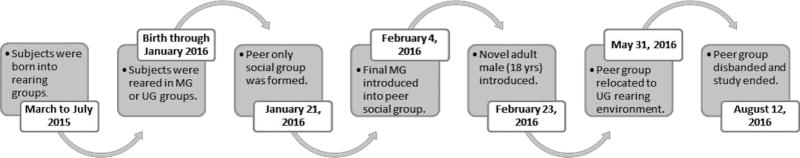
We studied 18 yearling rhesus macaques between January and August 2016 (see Figure 1). All subjects were born and reared at the Laboratory of Comparative Ethology (LCE) at the NIH Animal Center in Poolesville, Maryland, USA in the spring of 2015, with precisely known dates of birth. All subjects had ad libitum access to water and Purina Monkey Chow (#5038, St. Louis, MO). Fresh fruit and seeds were also provided twice daily. All procedures described adhered to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the NICHD Animal Care and Use Committee (ACUC).
Figure 1. Timeline of events throughout study.
2.1.1 Multi-generational (MG) subjects
Nine subjects (eight males, one female) were born and reared at the LCE field station (FS), which has been documented extensively in detail (Dettmer, Novak, Meyer, & Suomi, 2014; Dettmer, Woodward, & Suomi, 2015; Wooddell et al., 2016; Wooddell, Kaburu, Suomi, & Dettmer, 2017). Briefly, the FS was a 5-acre (2-ha) outdoor habitat, which also had access to three indoor runs (2.74 × 5.79 × 4.27m). The troop consisted of 80 semi-free ranging rhesus macaques (infants, juveniles, and adults) structured around naturally formed, MG matrilines that originated from the troop’s formation in the 1980s. The hierarchy was highly rigid, and offspring inherited the ranks of their mothers, with linear hierarchies evident in infancy (Wooddell, Kaburu, Suomi, & Dettmer, 2016).
Monkeys typically resided in the FS permanently, unless they were removed for health or social reasons such as rare contra-hierarchical fighting (e.g., Dettmer et al., 2015). In late December 2015, a rare overthrow occurred within the dominant matriline (Wooddell et al., 2017), resulting in hierarchical changes. Consequently, the nine MG subjects in this study, all from the previously dominant matriline, were permanently removed and placed together until this new social group with UG subjects was formed in January 2016.
2.1.2 Unigenerational (UG) subjects
Nine subjects (six males, three females) were born and reared into one of three UG harem groups consisting of 10–12 same-aged adult females, one adult male, and several same-aged infants (see Dettmer, Novak, Suomi, & Meyer, 2012; Dettmer et al., 2016). Infants born into the same harem group were paternal half-siblings. The groups lived in enclosures consisting of indoor (2.44 × 3.05 × 2.21 m) and outdoor (2.44 × 3.0 × 2.44 m) portions, equipped with perches, swings, barrels, and wood shavings.
As part of established protocols for our laboratory, UG infants were removed from their social groups at approximately 8-mos and placed into a social group with same-aged peers (see Dettmer et al., 2012). Typically, UG infants are placed with nursery-reared infants, but in this study owing to the unforeseen overthrow in the FS, the UG infants were grouped with the MG infants. This is therefore the first time a social group has been formed in our laboratory’s 30-plus year history of only mother-reared subjects, providing the unique opportunity to examine whether differences in early social experience (MG or UG), as well as maternal rank, would result in differential rank acquisition, as compared to the typical mother-rearing to nursery-rearing comparison examined in our laboratory (Bastian et al., 2003; Dettmer et al., 2012; 2016).
2.2 Yearling group formation and relocation
In January 2016, 17 subjects (eight MG and nine UG, all approximately 8-mos: mean age ± SEM: 274.44 ± 5.86 days) were introduced into a novel enclosure consisting of an indoor (7.3 × 3.4 × 3.7m) and outdoor portion (a circular corncrib measuring 5.03m in diameter by 5.49m high). Approximately 2-wks later, one final MG subject was introduced into the group after his mother’s unforeseen overthrow in the FS. A novel adult male (18-yrs) was introduced into the group 2-wks later as part of routine procedures in our laboratory to provide social interactions with an adult. Therefore, in total, the group consisted of 18 subjects and one adult male. Aside from the last MG subject introduced and the adult male, all were introduced to the run at the same time on the same day.
Unexpectedly, in late May 2016, the yearling group was relocated to one of the indoor/outdoor runs in which the UG subjects were initially reared (see UG rearing for housing conditions). The group remained there until it was disbanded in mid-August 2016. Thus, the group spent four months in a novel housing environment, followed by 2.5 months in a housing environment familiar to the UG monkeys only (see Figure 1).
2.3 Data collection
2.3.1 Mother’s dominance rank
Mothers’ dominance ranks were established via longitudinal data collection. In the FS, dominance data were recorded among all troop members via both focal and ad libitum sampling (Altmann, 1974) during routine coding. For mothers in the UG harem groups, dominance data were collected in two, 30-min sessions per week. In both conditions, all instances of aggression (threats, chases, attacks) and submission (displacements, fear grimaces) were recorded (see Dettmer et al., 2016; Wooddell et al., 2016). Ranks were established via Elo-rating (Neumann et al., 2011), a numerical system that continuously updates values based on wins and losses, which is especially beneficial in tracking rank changes over time (Wooddell et al., 2016; 2017). Each individual’s initial rating was set at 1,000, and the k factor was set at 200. The elo.sequence function (Neumann et al., 2011) generated Elo-ratings in R software (v 3.3.2). To control for differences in size between the different rearing groups, mother Elo-ratings were then transformed into relative ranks within their respective group by taking their ordinal rank divided by the total number of animals in their group. This was then subtracted from 1. Relative ranks therefore ranged from 0.07 (lowest-ranking) to 1 (highest-ranking). Mother relative ranks were calculated on the last day the subjects were with the mothers for UG subjects and the last day of data collection before the overthrow for the MG subjects. We also used the stability.index function (Neumann et al., 2011), which provides the S index where lower values reflect greater stability and higher values reflect greater instability (Neumann et al., 2011; Wooddell et al., 2017). We examined adult-adult interactions to assess the stability of the adult hierarchy while the subjects lived with their mothers prior to group formation. The S index for each of the UG harem groups was 0.004, 0.016, and 0.012, whereas the stability of the MG group was 0.264. The higher S index for the MG rearing group reveals that the FS troop had a higher degree of dominance instability prior to the overthrow (see also Wooddell et al., 2017).
2.3.2 Dominance rank in yearling group
During all data collection (dominance and focal sessions; see below), all individuals were separated to one half of the enclosure (inside portion or outside portion, balanced across days) to ensure that all individuals were visible. Dominance was collected in 30-min sessions in which all occurrences of aggression and submission were recorded three times per week by one observer (LJW). To gain the best representation of rank acquisition, we used all dominance interactions (decided/undecided and dyadic/polyadic). As part of a larger project, two, 5-min focal sessions were conducted on each subject per week by multiple observers (see below). During focal sessions, coders scored all aggressive and submissive interactions (as well as other social and nonsocial behaviors) involving the focal animal as well as all ad libitum dominance interactions within the rest of the group. To ensure the maximum amount of dominance data collected, focal sessions were never conducted at the same time as the primary dominance data collection. A total of 5,835 dominance interactions were collected during the study period. Elo-ratings (N=11,670; two for each interaction; one for the winner and loser) were used to construct dominance hierarchies over time, using the same procedure as the mothers’ ranks. We also used the stability.index function (Neumann et al., 2011) to examine rank changes following relocation.
2.3.3 Coalitions
A total of 631 coalitions were recorded during dominance data collection. Coalitions were defined as agonistic support given to one individual (either the winner or the loser) in a previous aggressive interaction. We also recorded “joint” coalitions, defined as two monkeys simultaneously aggressing another monkey, in which case it was not clear which monkey started the aggression and which one provided the support. For every coalition, the identities of all subjects were recorded, as well as whether the aider was supporting the winner or loser of the previous altercation. For analyses, we later calculated concurrent Elo-ratings for each individual in the coalition to examine what rank factors predicted coalitionary participation. Early social experience (MG or UG) was also later added to examine whether individuals supported individuals whom they were reared with.
2.3.4 Social affiliation
Focal behavioral data were collected via modified frequency sheets (Novak, Kinsey, Jorgensen, & Hazen, 1998) by three observers (AMM, AMD, LJW: inter-rater reliability ≥ 85%) via a 5-min continuous focal animal sampling method (Altmann, 1974). Each 5-min session was divided into 20, 15-sec intervals. Any behaviors that occurred within the 15-sec were recorded in chronological order. The maximum frequency a behavior could occur therefore was 20 intervals per session. In these sessions, all social and nonsocial behaviors were recorded for each focal subject. For the purposes of this study, we analyzed only social grooming (picking and spreading apart the fur) and social play (rough and tumble wrestling, play chasing, often accompanied by open-mouth play faces). The initiation of grooming was defined as the animal actively picking through the fur of another, whereas the recipient of grooming was the animal receiving the behavior. The initation of play was defined as the animal who solicited another peer for a play bout, whereas the receipt of play was defined as the individual who was solicited for the play bout. Each subject (N=18) was coded once in the morning (0900 to 1159) and once in the afternoon (1200 to 1700) each week. A total of 975 focal observations were collected (mean ± SEM: 54.14 ± 0.35 sessions per subject).
2.3.5 Body weights
Quarterly health exams were conducted in January (before group formation), April, and July 2016 where body weights were obtained.
2.4 Statistical analyses
Multiple linear regression was used to test whether individual traits such as weight (kg), age (days), sex (1=females, 2=males), and early social experience (1=MG, 2=UG), and maternal rank (relative social rank in her social group; ranged from 0.07 to 1) predicted significant variation in Elo-ratings (dependent variable) following group formation and group relocation. We reported the change in the R-squared value (ΔR2) of the model to evaluate each variable’s unique contribution to the explained variance in Elo-ratings. None of the independent variables were collinear (all VIF<1.5). Simple linear regression was used to access whether Elo-ratings on day 1 predicted later Elo-ratings.
In order to test whether subjects offered more coalitionary support to 1) winners/losers and 2) peers from the same or different early social experience group, we used paired t-tests (as data were normally distributed). We restricted the analyses on coalitionary supports directed to same vs different early social experience to peers, thereby excluding the adult male. Since the likelihood of supporting peers from the same early social experience group depends on how many peers were reared in the same or different group, we divided the number of coalitions directed to peers of the same or different group by the number of peers belonging to the two categories. We used the glmer function in the ‘lme4’ package in R 3.3.2 (Bates & Maechler, 2010) to run Generalized Linear Mixed Model (GLMM) with Poisson error structure (Zuur, Hilbe, & Ieno, 2013) in order to assess whether the absolute rank difference between the supporter and receiver as well as between supporter and opponent predicted the number of coalitions. To this end, we set the number of coalitions as the dependent variable and the absolute rank distance as the fixed factor, with the id of the subjects included as random factors. For each GLMM model we checked for lack of overdispersion and all the models showed either no or very little overdispersion (0.87 < φ <1.26).
Independent and paired sampled t tests were used to compare frequencies of coalitions, affiliation (grooming, play), and differences in Elo-ratings between and within early social experience groups respectively. All results are reported as mean ± SEM.
An average initiated and received score was calculated for each affiliative social behavior (grooming, play) for each subject to represent the average frequency an individual engaged in each of the social behaviors per 5-min session. The maximum frequency was 20 intervals. Therefore an average frequency of 4 indicated that the individual initiated or received that social behavior in 20% of the intervals (4/20). Spearman correlations were then used to test the associations between social behavior and rank both before and after the relocation, as well as rank changes (Elo-ratings following relocation minus Elo-ratings before relocation). We used Spearman correlations as the relationship between the two variables did not have a clear independent variable (social behavior can drive dominance rank or dominance rank can drive social behavior; but see Kohn et al., 2016; Snyder-Mackler et al., 2016 for how social rank drives social behavior).
Except where indicated, analyses were performed in SPSS 24 with alpha values set at P<0.05.
3. Results
Part I: Initial rank acquisition between group formation (January 19, 2016) and group relocation (May 30, 2016)
3.1 Ranks on day one of group formation
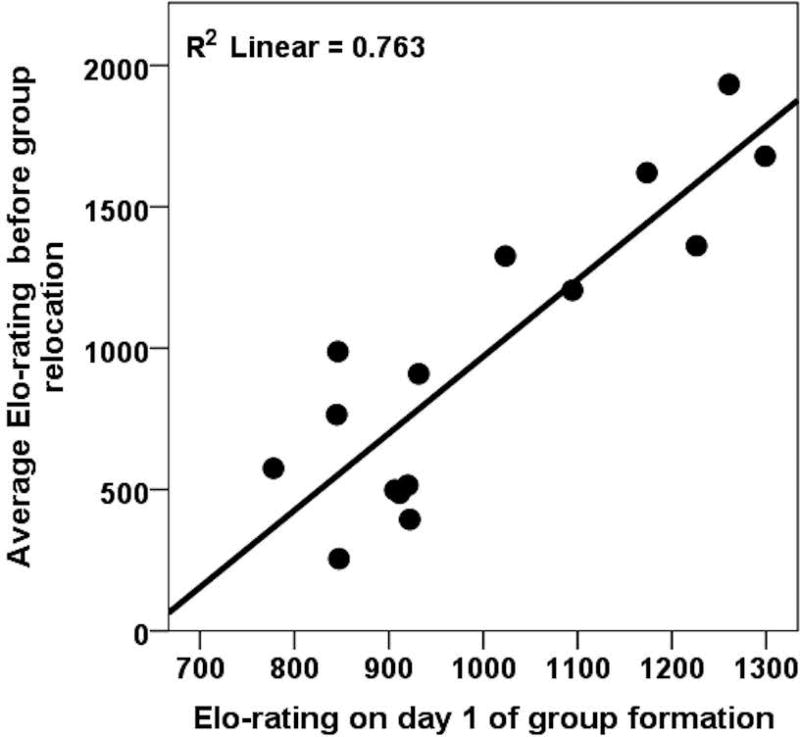
Following group formation, the hierarchy was rapidly established, with 15/17 (N=17 as the last MG subject was not yet introduced) subjects involved in dominance interactions (N=46 interactions) in the first 30-mins. Only early social experience explained a significant amount of variation in Elo-ratings on day one of group formation, with MG subjects outranking UG subjects (early social experience: ΔR2= 0.69, P<0.001; maternal rank: ΔR2=0.04, P=0.24, weight: ΔR2= 0.09, P=0.053; age: ΔR2= 0.003, P=0.73; sex: ΔR2= 0.00, P=0.99). The hierarchy on day one of group formation significantly predicted the hierarchy over the next few months before the relocation event (F(1,13)=41.77, P<0.001, R2=0.76, β=0.87, see Figure 2), suggesting that the group hierarchy was formed in less than an hour and was relatively stable for the next four months until relocation.
Figure 2. Elo-rating on day one of group formation and over time.
Elo-ratings on day one of group formation of a group of rhesus macaque yearlings predicted Elo-ratings over the next few months, suggesting that a hierarchy was rapidly established.
3.2 Did individual traits predict rank acquisition?
Extending out past day one (day two until relocation four months later), none of the individual traits significantly related to rank acquisition in this group of yearlings. Specifically, weight at group formation (ΔR2=0.00, P=0.98) age (ΔR2=0.02, P=0.43), and sex (ΔR2=0.005, P=0.63), did not add any significant predictive value to the model for Elo-ratings before the relocation event. These findings supported prediction 1.
3.3 Did social experience predict rank acquisition?
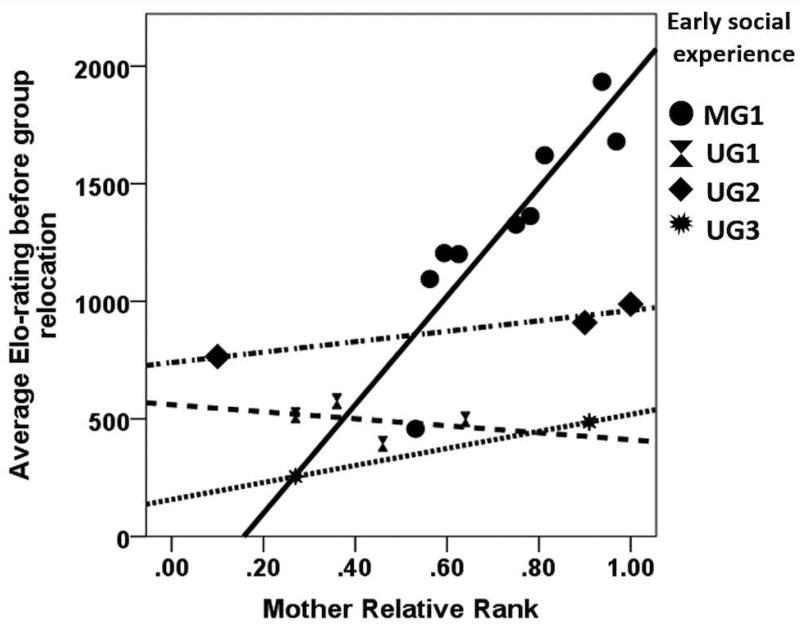
Contrary to the individual traits, social experience explained a significant portion of the variance in the model for rank acquisition in this group of rhesus macaque yearlings. Both early social experience (MG vs UG) and maternal rank added significant predictive value to the model for Elo-ratings (early social experience: ΔR2= 0.55, P<0.001; maternal rank: ΔR2= 0.13, P=0.02, see Figure 3; supporting predictions 2 and 3), although early social experience explained more of the variation than maternal rank. This difference can be explained by the fact that not all UG subjects were reared together (as they were born into one of three separate harem groups; however all MG subjects were reared together in one large troop). Indeed, when comparing MG to UG subjects, the relationship between maternal rank and offspring rank was only significant for MG subjects (MG: (F(1,7)=24.25, P=0.002, R2=0.78, β=0.88; UG: F(1,7)=2.05, P=0.20, R2=0.23, β=0.48).
Figure 3. Relationship between maternal rank and offspring rank.
A mother’s relative rank in her social group positively predicted her offspring’s rank following the offspring’s permanent removal and introduction into a new social group of yearlings, although this was primarily driven by subjects who were previously reared together, such as the MG peers.
One interesting case was the last MG subject who was introduced approximately 2-wks after the group had been formed. Upon group entry, all subjects quickly submitted to him (even his previous MG peers), although he directed no aggression. However in the following days, he became the target of aggression and quickly descended to the lowest-ranking animal in the group, even ranking below the UG subjects. He remained the lowest-ranking in the group throughout the study.
3.4 Role of coalitions in rank acquisition
MG subjects did not initiate more coalitionary support than UG subjects (MG: 11.78 ± 2.36; UG: 6.44 ± 1.73; t(16)=−1.82, P=0.087), although this finding was likely influenced by the last MG subject who was introduced into the group 2-wks after group formation, obtained the lowest ranking position, and initiated no coalitionary support. When we excluded this subject, MG subjects initiated significantly more coalitionary support than UG subjects (MG: 13.25 ± 2.09; UG: 6.44 ± 1.73; t(15)=−2.52, P=0.02; supporting prediction 4a). There was no significant difference in received coalitionary support between MG and UG subjects, with (MG: 11.78 ± 1.60, UG: 10.11 ± 2.33; t(16)=−0.59, P=0.56) or without (MG: 12.5 ± 1.61, MPR: 10.11 ± 2.33; t(15)=−0.84, P=0.41) the last MG subject. No joint coalitions (multiple animals aggressing another individual simultaneously) were observed prior to relocation.
Individuals were more likely to support peers from the same rearing group (either their harem UG rearing group or the MG FS troop), supporting prediction 4b (paired t-test: t (16)=4.40, P<0.001). Additionally, prior to relocation, the number of coalitions was not significantly predicted by the absolute rank distance between the two coalitionary partners (Poisson GLMM: Estimate ± SE = 0.02 ± 0.02; z=0.90, P=0.36). However, there was a negative relationship between the number of coalitions and rank distance between the supporter and opponent (Estimate ± SE = −0.04 ± 0.02; z=−1.97, P=0.048), with coalitions occurring more frequently when the opponent was close in rank. Furthermore, individuals were more likely to support the winners in coalitions (t (16)=−2.74, P=0.01), suggesting again that coalitions were likely a mechanism to reinforce the hierarchy (supporting prediction 4a).
Additionally, the adult male preferentially supported the three UG females, who spent more time in social proximity to him (data not shown). Out of 34 interventions initiated by the adult male, 29 of them (85%) supported one of these three females following their loss in an aggressive interaction. These three females became the top-ranking animals in the group following relocation.
3.5 Did social affiliation relate to rank acquisition?
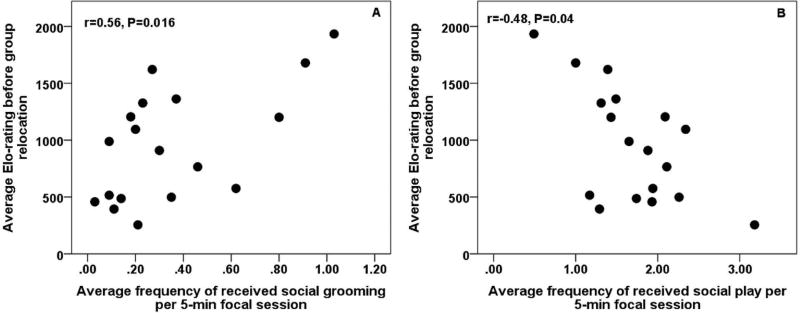
There were no significant differences between initiated grooming or initiated play between MG and UG subjects (initiated grooming: MG: 0.69 ± 0.21, UG: 0.43 ± 0.13, t(16)=−1.02, P=0.32; initiated play: MG: 1.59 ± 0.24, UG: 1.76 ± 0.12; t(16)=0.62, P=0.55). The initiation of grooming (rs=0.01, P=0.97, N=18) and social play (rs=0.24, P=0.34, N=18) were not significantly correlated with average Elo-rating before relocation, failing to support prediction 5. However, Elo-rating was positively correlated with receiving social grooming (rs=0.56, P=0.016, N=18; see Figure 4a) and negatively correlated with receiving social play (rs=−0.48, P=0.04, N=18; see Figure 4b).
Figure 4. Social affiliation and rank acquisition.
High ranking rhesus macaque yearlings received more frequent social grooming (a) but less frequent social play (b) following peer group formation.
Part II: Rank acquisition and rank changes between group relocation (May 31, 2016) and group disbandment (August 12, 2016)
3.6 Relocation event and rank changes
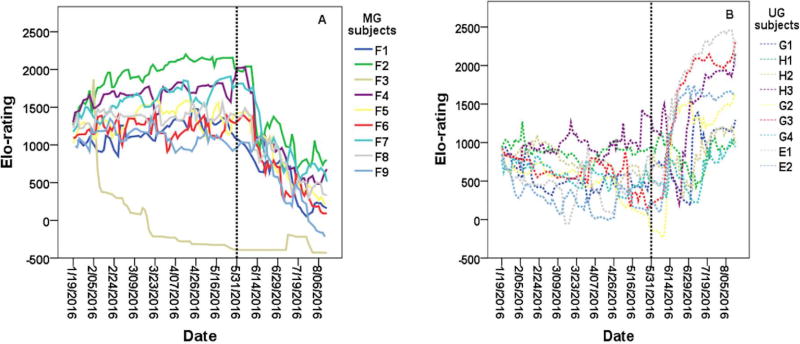
On May 31, 2016, the social group was relocated to the physical rearing environment of the UG subjects. No changes in the hierarchy occurred on the day of relocation. However, on the 13th of June, clear changes in the hierarchy had emerged. 143 dominance interactions were recorded in only 30-mins, with a previously low-ranking female (E1) now clearly established as the alpha yearling. The Elo-ratings on the first day of overt rank reversals (13th of June) significantly predicted the hierarchy over the next few months (F(1,16)=10.21, P=0.006, R2=0.39, β= 0.62). However, it is important to note that rank changes continued to occur over the following months, with a slow progression of the lowest ranking UG subjects outranking the MG subjects. The stability index increased from 0.17 to 0.40 in the month following the relocation, suggesting higher levels of instability and rank changes. While housed in this new environment, the MG subjects experienced a significant decrease in Elo-ratings (mean Elo-rating, before= 1320.13 ± 140.46; after=624.04 ± 146.49; t(8)=25.12, P<0.001; see Figure 5a), whereas the UG subjects experienced a significant increase in Elo-ratings (mean Elo-rating, before = 598.57 ± 80.44; after= 1163.65 ± 133.63; t(8)= −3.14, P=0.01; see Figure 5b), supporting prediction 6.
Figure 5. Rank changes in a group of rhesus macaque yearlings following relocation to a familiar environment.
The MG subjects (a) descended in rank below the UG subjects (b) following the group’s relocation (black dotted vertical line) to the rearing environment of the UG subjects.
3.7 Did individual traits predict rank following relocation?
Weight in April (ΔR2=0.004, P=0.74), age (ΔR2=0.10, P=0.11), and sex (ΔR2=0.06, P=0.22) did not add any significant predictive value to the model for Elo-ratings following relocation. It is however important to note that the top three animals were now all UG females, who had strong alliances with one another and the adult male.
3.8 Did social experience predict rank following relocation?
Early social experience (MG or UG) significantly explained the variation in Elo-ratings following group relocation (ΔR2=0.32, P=0.015), but a reversal occurred such that UG subjects now outranked all MG subjects, suggesting that previous social experience in this physical environment was important (supporting prediction 6). The hierarchy among MG peers remained highly stable, as Elo-ratings following group formation positively predicted Elo-ratings following group relocation (MG hierarchy: F(1,7)=191.59, P<0.001, R2=0.97, β= 0.98). Post-relocation, MG yearlings’ ranks were still heavily influenced by maternal rank (MG: F(1,7)=15.57, P=0.006, R2=0.69, β=0.83). This suggests that while the MG subjects now descended in rank below the UG subjects, their hierarchy still remained stable among one another, contingent upon maternal rank. For UG subjects, Elo-ratings following group formation did not predict Elo-ratings following group relocation (UG hierarchy: F(1,7)=1.16, P=0.32, R2=0.14, β=−0.38), and neither did maternal rank (UG hierarchy: F(1,7)=0.16, P=0.70, R2=0.02, β=0.15), even within each harem group. These findings indicate that UG subjects not only ascended in rank above the MG subjects, but ranks among the UG subjects changed, even within their rearing harem groups. In addition, bidirectional aggression, which was defined as two animals aggressing one another with no clear winner and observed as violent retaliated aggression and a clear challenge to the dominance position, increased from 4 to 33 occurrences following relocation. These interactions occurred mainly between UG and MG dyads (indicating challenges from the UG subjects to the MG subjects: N=22) and less often between UG-UG dyads (N=11), especially those that were reared together (indicating challenges within the UG harem rearing groups; N=10/11). No MG dyads ever engaged in bidirectional aggression with one another.
3.9 Role of coalitions in rank acquisition following relocation
Following relocation, UG subjects had a significant increase in the levels of initiated coalitionary support compared to before relocation (before relocation= 6.44 ± 1.73; after relocation= 31.67 ± 4.44; t(8)=4.91, P=0.001), as well as received coalitionary support (before relocation= 10.11 ± 2.33; after relocation= 31.89 ± 4.67; t(8)=5.36, P=0.001). MG subjects however had no significant change in the levels of initiated (before relocation= 11.78 ± 2.36; after relocation=13.89 ± 3.69; t(8)=0.60, P=0.57) or received (before relocation= 11.78 ± 1.60; after relocation=14.89 ± 1.98; t(8) =1.88, P=0.097) levels of coalitionary support. UG subjects also initiated and received significantly more coalitionary support than their MG peers following relocation (initiated: t(16)=3.08, P=0.007; received: t(16)=10.80, P=0.007).
Joint coalitions increased tremendously (before relocation= 0 ± 0; after relocation= 9.05 ± 1.42; t(17)=6.38, P<0.001), for both UG (before=0 ±0; after=11.33 ± 2.50; t(8)=4.53, P=0.002) and MG subjects (0 ± 0; after=7.22 ± 1.42; t(8)=5.08, P=0.001), although there was no significant difference between MG and UG subjects (t(16)=1.43, P=0.17). Joint coalitions were typically observed as mobbing events, with multiple animals severely aggressing one another.
Similar to the period before relocation, individuals were more likely to support winners in coalitions (t(17)=−4.27, P<0.001), as well as individuals from the same rearing group (t(16)=5.43, P<0.001). Moreover, there was a negative relationship between the number of coalitions and rank distance between aider and recipient (Estimate ± SE = −0.07 ± 0.02, z=−4.2, P<0.001), suggesting that coalitions primarily occurred between individuals close in rank. Additionally, contrary to prior to relocation, there was a positive relationship between the number of coalitions and the distance between the aider and the opponent (0.03 ± 0.02, z=2.01, P=0.04), suggesting that aiders engaged in coalitions when the opponent was far in rank.
On the last day of data collection, the alpha female received joint aggression from the beta and gamma females (both UG) who had a very strong alliance with each other. The alpha female dropped to the #3 rank on the last day of data collection (see Figure 5b; monkey E1).
4.0 Did social affiliation relate to rank following relocation?
Following relocation, there was a significant increase in the frequency of initiated social grooming (before=0.56 ± 0.13, after= 1.89 ± 0.28; t(17)=−4.91, P=<0.001) and a significant decrease in the frequency of initiated social play (before= 1.67 ± 0.13, after= 0.84 ± 0.14; t(17)=6.55, P<0.001). In addition, there was a nearly significant difference in the frequency of initiated social grooming between MG and UG subjects, with MG subjects grooming more than UG subjects (MG: 2.37 ± 0.48, UG: 1.41 ± 0.23; t16= −1.81, P=0.08), and a significant difference in the frequency of initiated social play, with UG subjects playing more than MG subjects (MG: 0.55± 0.14, UG: 1.13 ± 0.22; t(16)= 2.19, P=0.04). No initiated social behavior (initiated grooming: rs=−0.20, P=0.42, N=18; initiated play: rs=0.20, P=0.42, N=18) or received social behavior (received grooming: rs=−0.18, P=0.49, N=18; received play: rs=−0.13, P=0.62, N=18) significantly correlated with Elo-ratings following relocation.
4.1 Social play and rank changes
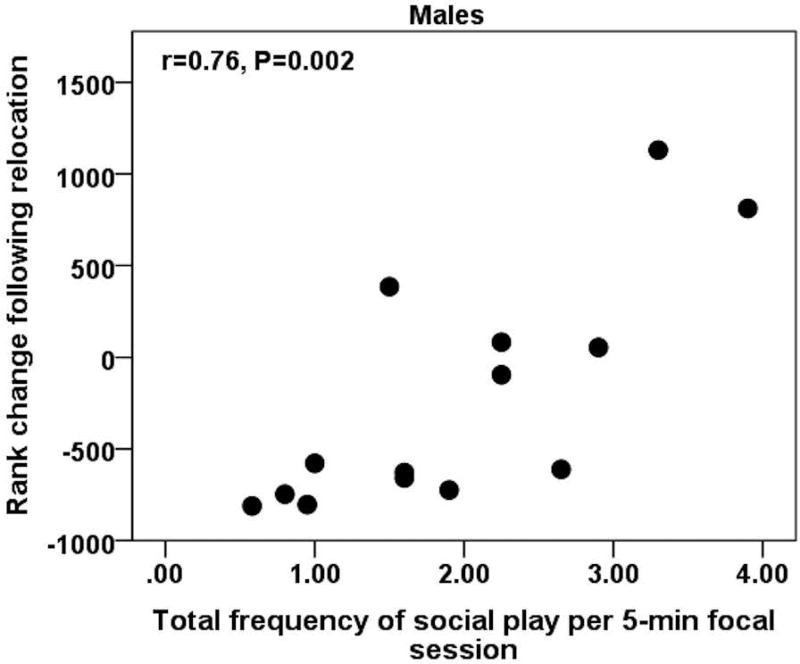
Given that social play can be seen as “a practicing behavior” that is not contingent on the initiation or receipt of the play bout, we calculated the total frequency of social play (both initiated and received) following relocation. We then correlated this with rank change (Elo-ratings after relocation – Elo-ratings before relocation) to examine whether individuals that engaged in more social play were more likely to achieve higher ranks following social relocation and instability. When doing so, we found that males who engaged in higher frequencies of social play overall also had significantly higher rank increases following relocation (males: rs=0.76, P=0.002, N=14, see Figure 6; females: rs=0.40, P=0.60, N=4).
Figure 6. Social play and rank changes following relocation in a peer group of rhesus macaque yearlings.
Males who engaged in higher frequencies of social play per 5-min session also had higher levels of rank changes following relocation.
5. Discussion
We sought to explore the factors that predicted rank acquisition in a newly established group of rhesus macaque yearlings, all of which were reared with their mothers and peers for the first 8-mos of life, albeit in environments that differed in social and physical complexity. We analyzed individual traits such as weight, age, and sex and social predictors such as early social experience, maternal rank, coalitionary support, and social affiliation (grooming and social play), as well as the physical environment. We found that social factors, but not individual traits, as well as familiarity with a physical environment, were related to the acquisition of dominance rank in yearlings. Given the species-typical method of rank inheritance of rhesus and Japanese macaques (Kawai, 1958), our results suggest that certain mechanisms of rank inheritance can persist in peer-only social groups (Jenks, Weldele, Frank, & Glickman, 1995), even following permanent maternal separation. These results provide exciting insights into the mechanisms of rank acquisition among peers.
Unsurprisingly, individual traits such as weight, age, and sex were unrelated to dominance acquisition in this study. Similarly, a previous study conducted in our laboratory found that while weight at group formation (8-mos) did not predict later juvenile dominance rank at 3-yrs, weight taken at the end of the study correlated with dominance ranks (Bastian et al., 2003), suggesting that dominance rank influenced subsequent weight gain, rather than weight influencing subsequent dominance rank. More importantly, in the current study, the weight and age differences (max=0.5 kg and 108 days) between the individuals were minor, and therefore the influences of age and weight may have more pronounced effects in more diverse groups. In addition, sex did not predict rank acquisition, but our heavily skewed ratio of male subjects (14 males; 4 females) precluded us from drawing any strong conclusions about possible sex differences in rank acquisition. It is important to note that the top three animals following relocation were all females, although this was influenced by rearing, as all top three females were UG (the only other female in the group was MG). Intriguingly, a study with human toddlers also found that females tended to be more dominant than males (Hawley & Little, 1999), in which the authors hypothesized was due to the lack of differences in aggression before the age of three (Maccoby & Jacklin, 1980; rhesus macaques: Kulik, Amici, Langos, & Widdig, 2015), which is typically the age in which males become more dominant (LaFreniere & Charlesworth, 1987). In addition, Hawley & Little (1999) hypothesized that females may use “prosocial strategies” to attain social dominance, whereas males may rely more on direct interactions. In Assamese macaques (Macaca assamensis), for example, some females form strong bonds with males, thus receiving protection from harassment from other group members (Haunhorst, Heesen, Ostner, & Shülke, 2017), while other studies have reported that affiliative relationships among low-ranking juvenile females and top-ranking monkeys (males and females) were related to the acquisition of unusually high ranks even in the absence of direct support in conflicts (Ball, 1997; Small, 1990), thereby demonstrating the utilization of very effective “prosocial” strategies. In our study, we found that prior to relocation, the three UG females spent a large amount of time around the adult male (data not shown), resulting in his support, presumably because the harassment occurred in close proximity. While we did not observe the adult male aiding the females in rank challenges following relocation, it is possible that his prior support reduced the likelihood of retaliated aggression from the MG peers during the rank challenges. Future research should address possible different strategies in rank acquisition for males and females, as our study was unable to do so with the low sample size of females (N=4).
We also found that early social experience significantly influenced initial rank acquisition, with subjects reared in a MG, naturalistic population outranking those reared in a captive, UG population. We have previously found that mother-reared infants obtain higher ranks than nursery-reared infants (Bastian et al., 2003; Dettmer et al., 2016), and here we demonstrate differences due to the complexity of mother rearing. There are a few hypotheses that could explain why the MG peers may have been more dominant following initial group formation. One hypothesis is that the MG subjects had more collective social power than the UG subjects, as all nine of the MG subjects were reared together, resulting in increased odds for coalitionary support, compared to the UG subjects who were reared with 1–3 other peers. In addition, the MG subjects were reared in a more complex social environment, which may have promoted social competency, especially since all subjects were from the dominant matriline. Similarly, the MG subjects endured their mothers’ overthrows in the FS, and these subjects may have quickly asserted their dominance once relocated to the new social group. However, it is important to note that the last MG subject introduced approximately 2-wks later did not rank immediately after the MG subjects as would be expected, but instead ranked at the bottom of the hierarchy, even below the UG subjects. The order of introduction therefore annulled the impacts of early social experience and kin support, similar to a study in willow tits (Parus montanus), in which the residency effect overruled the impacts of body size (Koivula, Lahti, Orell, & Ryktönen, 1993). Tenure in a social group may therefore have even greater effects on rank acquisition than other established factors. Nevertheless, our results suggest that future research should not only investigate the outcomes of varying maternal rearing conditions but also possible differences in individual maternal behavior. This will then allow us to address whether differential levels of maternal care and investment result in differential rank acquisition.
Maternal rank also influenced rank acquisition. In particular, subjects that were previously reared together obtained identical relative ranks as their mothers, suggesting that maternal rank inheritance persisted even in the absence of the mother (Jenks et al., 1995; Loy & Loy 1974). We previously found in our FS troop that maternal rank predicted offspring’s rank for all age categories: infancy (<1 yr), yearlings, 2-yrs, and 3-yrs, with offspring typically ranking adjacent to their mother by 3-yrs (Wooddell, Kaburu, Suomi, & Dettmer, 2016). Our previous results found that even infants established a linear hierarchy among themselves contingent on maternal rank, although mothers and other kin were available for support. The results from the current study suggest that maternal rearing for the first 8-mos of life is enough time for infants to learn their relative ranks among one another, even following permanent maternal separation, indicating that maternal presence is likely necessary for initial rank acquisition but not rank maintenance. The stability of maternal rank inheritance was especially evident for the MG subjects, as their hierarchy remained stable throughout the entire study, whereas the UG subjects endured some rank changes. Loy & Loy (1974) also found that a juvenile group of rhesus monkeys retained their matrilineal ranks when separated from their matriline on Cayo Santiago. As they eloquently describe, “The juveniles did not fall into behavioral chaos, but rather maintained that organization and those relationships which they had known as a segment of the larger unit” (Loy & Loy, 1974, page 94). We hypothesize that in large MG groups, ranks are more heavily reinforced and less flexible, as numerous opportunities for kin support (mothers, sisters, aunts, grandmothers, cousins, etc) persist in these groups, especially compared to UG groups in which the only regular form of kin support is from the mother. This could help explain why the MG hierarchy remained highly rigid, although the hierarchies among UG subjects were slightly challenged. Yet, it is also possible that the tenure of the social groups (30+ years for the FS troop, 7, 8, & 10 years for the three UG harem groups) may also have been a factor, as long established groups, regardless of the complexity of generations, may promote social competency and rank acquisition. Regardless, the maintenance of an already established hierarchy for the MG subjects was presumably less stressful than a complete social reorganization (i.e., if the MG subjects had also challenged each other) as the persistence of some stable relationships is likely better than complete chaos and no stable relationships. Indeed, even in matrilineal overthrows, there often is a dominance succession pattern where individuals may just simply move up in the hierarchy following the overthrow of alpha families (Wooddell et al., 2017), and entire matrilines can remain stable in their ranks during these social upheavals (Ehardt & Bernstein, 1986; Wooddell et al., 2017). Even during social reorganization, certain groups can remain stable in their ranks among one another, which has some inherent benefit, such as limited changes in infant mortality for uninvolved matrilines following a matrilineal overthrow (Dettmer et al., 2015) or potentially glucocorticoid production during matrilineal instability (Wooddell et al., 2016). Future research should address the possible benefits of individuals that retain a stable hierarchy during a period of social reorganization.
Finally, the relatively long process of rank acquisition in naturalistic populations is likely due to the slower process of ascension above older animals, rather than peers. Our results, and the results of other studies, suggest that hierarchies among peers develop first (although see Sandel, Reddy, & Mitani, 2017 in chimpanzees). Indeed, de Waal & Luttrell (1985) also found that bared-teeth displays (also known as fear grimaces), a formal signal of subordination, developed among peers relatively quickly, followed by the slower transition to older matriarchs. The speed of rank acquisition is thus likely a process contingent upon the demographics of an individual’s own social group.
Coalitions were another mechanism yearlings used to maintain dominance rank, and we found evidence for nepotism, or kin bias, as coalitions were more frequent among peers that were reared together (and therefore either maternally or paternally related). Similarly, a recent study also found support for post-dispersal nepotism in male long-tailed macaques (Macaca fascicularis), as males that entered into a troop with relatives resided in that troop and maintained a higher rank for longer (Gerber et al., 2016), suggesting that kin support continues in new social groups, similar to our results. Our results also suggest that coalitions were primarily a mechanism to reinforce, rather than challenge, the hierarchy (Smith et al., 2010). Coalitions were more frequent among high-ranking monkeys (the MG subjects prior to relocation; UG subjects following relocation), and supporters were more likely to aggress losers of the previous interaction, thereby supporting the winner and promoting a winner-loser effect. While we found no significant rank-related relationship between the two coalitionary partners prior to relocation, post-relocation, we found that individuals that were closely ranked were more likely to aid each other in coalitions, signaling again that the coalition reinforced both of their ranks relative to the opponent, thereby benefiting them both. Moreover, we found different rank-related relationships between the supporter and the opponent before and after relocation. Prior to relocation, individuals were more likely to engage in coalitions when the opponent was close in rank, suggesting that individuals engaged in “risky” coalitions. Following relocation, a period of instability ensued, and accordingly, supporters engaged in coalitions when the opponent was substantially lower in rank. Why might individuals engage in coalitions when the opponent was a closely matched competitor prior to relocation but a distantly ranked competitor after relocation? This perhaps has to do with the stability of the group. During stability, individuals may engage in coalitions to reinforce their position to closely ranked competitors, which are the ones who typically pose the greatest threat, thereby maintaining stability. In periods of social instability, in which ranks are unsettled, coalitions may be more frequent against distantly ranked opponents to minimize the costs to themselves in order to reinforce the status quo. Our results add to the growing literature suggesting that participation in coalitionary support is a flexible decision making process, one that can be adapted to the unique social and environmental circumstances.
Additionally, we found unexpected results regarding social affiliation (grooming and play) and dominance rank. While we found no evidence that the initiation of grooming or play was significantly correlated with rank before or after relocation, we did find that higher ranking yearlings received grooming more frequently and social play less frequently. These results both intuitively suggest that social affiliation was likely a product rather than a determinant, of social rank (see also Snyder-Mackler et al., 2016). Moreover, given that social play can quickly escalate to physical aggression if the bout becomes too rough, high ranking monkeys may be less preferred play affiliates, explaining why we actually found a negative, rather than positive, relationship between received play and rank. Furthermore, no significant results may have been found following relocation because ranks were likely not fully established (as even on the last day of data collection, the alpha yearling was outranked). In addition, our results revealed that during a period of social instability, grooming increased, which we have previously found in our adults in the FS troop during instability (Wooddell et al., 2016). Grooming may therefore be a mechanism to reduce social tension (Judge, Griffaton, & Fincke, 2006; Schino, Scucchi, Maestripieri, & Turillazzi, 1988), even in yearlings. Intriguingly, social play conversely decreased during a period of social instability, suggesting that play occurs when environmental and social conditions are stable (Barrett, Dunbar, & Dunbar, 1992). While we did find that males who played more had significantly higher rank changes following relocation, these results should be regarded with caution given that the relationship between social play and dominance rank remains contested (relationship: Blumstein et al., 2013; Paquette, 1997; no relationship: Perry, Godoy, Lammers, & Lin, 2017; Sharpe, 2005). Perhaps social play is a mechanism to assess the competitive strength of others and refine motor skills during the period of social instability, thereby promoting higher rank changes, or perhaps social play is simply a byproduct of rank ascension. The mechanisms by which social play can attenuate the influences of rank changes during a period of social instability thus warrant future research.
Finally, perhaps the most intriguing result from this study was the dramatic rank ascension of the UG subjects following the group’s relocation to their rearing environment. Familiarity with the environment can therefore disrupt even established dyadic rank relationships. Angermeier, Phelps, Murray, & Reynolds (1967, page 434) similarly concluded, “When two monkeys are tested with one monkey from a different home environment, like living condition seems to be the single most important factor in the establishment of dominance”. It is possible that the familiarity with the environment reduced the stress response in the UG subjects (compared to the MG subjects) following relocation, allowing the UG subjects to allocate relatively more time to social play, dominance, and coalitions (Nezlek, 2007; Morales, Varlinskaya, & Speark, 2013; Varlinskaya & Spear, 2002; Wattanakulab, Edwardsa, Stewarta, & Englishb, 1998; Wilson, 2001), thereby promoting rank increases. We are currently analyzing hair cortisol concentrations before and after group formation and relocation, which will add to our knowledge about the role of environmental familiarity in relation to social behavior, rank changes, and chronic hypothalamic-pituitary-adrenocortical (HPA) axis activity. Finally, the relocation itself was likely not the catalyst to the rank changes, but the relocation to a familiar environment, as relocations to novel environments may only result in limited rank changes (Honess, Johnson, & Wolfensohn, 2004). While not possible in this study, ideally we would have relocated this group back to the initial housing environment following peer formation to examine if this would again result in hierarchical changes, in which the MG monkeys would again be dominant. Furthermore, our results add to the growing body of literature suggesting that dominance is a fluid relationship, specific to the social and physical environment (Bernstein & Gordon, 1980).
Unfortunately, our study was unable to follow the group longitudinally into juvenility, adolescence, and adulthood to examine rank (in)stability through varying ages, hormonal changes, alliance shifts, and behavioral and physiological development. We thus encourage future research to address rank acquisition longitudinally in newly established groups. In addition, we acknowledge that social overthrows may result in relocations that promote rank acquisition atypical of naturalistic processes and that our sample size was small due to the unique circumstances.We encourage future research to investigate the influence of MG social experience on subsequent rank acquisition in novel groups without the occurrence of a social overthrow.
In conclusion, our study highlights the importance of social mechanisms and the physical environment on rank acquisition in rhesus macaque yearlings following permanent maternal separation. Future research should address rank acquisition in peer groups without previous social experience with one another to determine the factors that predict rank acquisition with completely unfamiliar individuals. This will add to the growing body of literature detailing the multi-faceted process of rank acquisition, which has behavioral and physiological outcomes.
Acknowledgments
This research was supported by the Division of Intramural Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. We thank the animal care and veterinary staff at the NIH Animal Center for their dedicated care and treatment to the monkeys.
Footnotes
The authors have no conflicts of interest to declare.
References
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49(3):227–266. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Altmann J. Baboon Mothers and Infants. Cambridge: Mass. Harvard University Press; 1980. [Google Scholar]
- Angermeier WF, Phelps JB, Murray S, Reynolds HH. Dominance in monkeys: early rearing and home environment. Psychonomic Science. 1967;9(7):433–434. [Google Scholar]
- Ball HL. Daddy’s girl? Anomalous social rank of a female rhesus macaque (Macaca mulatta) Folia Primatologica. 1997;68:44–49. doi: 10.1159/000157231. [DOI] [PubMed] [Google Scholar]
- Barrett L, Dunbar RIM, Dunbar P. Environmental influences on play behaviour in immature gelada baboons. Animal Behaviour. 1992;44(1):111–115. [Google Scholar]
- Bastian ML, Sponberg AC, Suomi SJ, Higley JD. Long-term effects of infant rearing condition on the acquisition of dominance rank in juvenile and adult rhesus macaques (Macaca mulatta) Developmental Psychobiology. 2003;42:44–51. doi: 10.1002/dev.10091. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M. Package ‘lme4.’. 2010 [ http://lme4.r-forge.r-project.org/]
- Bernstein IS, Gordon TP. The social component of dominance relationships in rhesus monkeys (Macaca mulatta) Animal Behaviour. 1980;28:1033–1039. [Google Scholar]
- Bernstein IS, Ehardt CL. Agonistic aiding: kinship, rank, age and sex influences. American Journal of Primatology. 1985;8:37–52. doi: 10.1002/ajp.1350080105. [DOI] [PubMed] [Google Scholar]
- Boulton MJ. Rough physical play in adolescents: does it serve a dominance function? Early Education and Development. 1992;3(4):312–333. [Google Scholar]
- Blumstein DT, Chung LK, Smith JE. Early play may predict later dominance relationships in yellow-bellied marmots (Marmota flaviventris) Proceedings of the Royal Society of Britain. 2013;280 doi: 10.1098/rspb.2013.0485. 20130485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapais B. Rank maintenance in female Japanese macaques: experimental evidence for social dependency. Behaviour. 1988a;104:41–59. [Google Scholar]
- Chapais B. Experimental matrilineal inheritance of rank in female Japanese macaques. Animal Behaviour. 1988b;36:1025–1037. [Google Scholar]
- Cheney DL. The acquisition of rank and the development of reciprocal alliances among free-ranging immature baboons. Behavioral Ecology and Sociobiology. 1977;2(3):303–318. [Google Scholar]
- de Waal F. Chimpanzee politics: Sex and power among apes. London, UK: Jonathan Cape; 1982. [Google Scholar]
- de Waal FDM, Luttrell LM. The formal hierarchy of rhesus macaques: an investigation of the bared-teeth display. American Journal of Primatology. 1985;9(2):73–85. doi: 10.1002/ajp.1350090202. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Suomi SJ, Meyer JS. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37(2):191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Novak M, Meyer J, Suomi SJ. Population density-dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta) Psychoneuroendocrinology. 2014;42:59–67. doi: 10.1016/j.psyneuen.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Woodward RA, Suomi SJ. Reproductive consequences of a matrilineal overthrow in rhesus monkeys. American Journal of Primatology. 2015;77(3):346–352. doi: 10.1002/ajp.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Wooddell LJ, Rosenberg KL, Kaburu SKK, Novak MA, Meyer JS, Suomi SJ. Associations between early life experience, chronic HPA axis activity, and adult social rank in rhesus monkeys. Social Neuroscience. 2016;12(1):92–101. doi: 10.1080/17470919.2016.1176952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East ML, Höner OP, Wachter B, Wilhelm K, Burke T, Hofer H. Maternal effects on offspring social status in spotted hyenas. Behavioral Ecology. 2009 arp020. [Google Scholar]
- Ehardt CL, Bernstein IS. Matrilineal overthrows in rhesus monkey groups. International Journal of Primatology. 1986;2:157–181. [Google Scholar]
- Foerster S, Franz M, Murray CM, Gilby IC, Feldblum JT, Walker KK, Pusey AE. Chimpanzee females queue but males compete for social status. Scientific reports. 2016;6:35404. doi: 10.1038/srep35404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazes RG, Hampton RR, Lourenco SF. Transitive inference of social dominance by human infants. Developmental Science. 2015;18:1–10. doi: 10.1111/desc.12367. [DOI] [PubMed] [Google Scholar]
- Georgiev AV, Christie D, Rosenfield KA, Ruiz-Lambides AV, Maldonado E, Emery Thompson M, Maestripieri D. Breaking the succession rule: the costs and benefits of an alpha-status take-over by an immigrant rhesus macaque on Cayo Santiago. Behaviour. 2016;153:325–351. [Google Scholar]
- Gerber L, Krützen M, de Ruiter JR, van Schaik CP, van Noordwijk MA. Postdispersal nepotism in male long-tailed macaques (Macaca fascicularis) Ecology and Evolution. 2016;6(1):46–55. doi: 10.1002/ece3.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LH, Trancik A, Bensadoun J, Boyce WT, Alder NC. Social dominance and cardiovascular reactivity in preschoolers: associations with SES and health. Annals of the New York Academy of Sciences. 1999;896:363–366. doi: 10.1111/j.1749-6632.1999.tb08142.x. [DOI] [PubMed] [Google Scholar]
- Gouzoules H. Maternal rank and early social interactions of infant stumptail macaques, Macaca arctoides. Primates. 1975;16(4):405–418. [Google Scholar]
- Haunhorst CB, Heesen M, Ostner J, Shülke O. Social bonds with males lower the costs of competition for wild female Assamese macaques. Animal Behaviour. 2017;125:51–60. [Google Scholar]
- Harcourt AH, de Waal FB. Coalitions and alliances in humans and other animals. Oxford University Press; 1992. [Google Scholar]
- Hawley PH, Little TD. On winning some and losing some: a social relations approach to social dominance in toddlers. Merrill-Palmer Quarterly. 1999;45(2):185–214. [Google Scholar]
- Holand Ø, Gjøstein H, Losvar A, Kumpula J, Smith ME, Røed KH, Nieminen M, Weladji RB. Social rank in female reindeer (Rangifer tarandus): effects of body mass, antler size and age. Journal of Zoology. 2004;263(4):365–372. [Google Scholar]
- Holekamp KE, Smale L. Dominance acquisition during mammalian social development: the “inheritance” of maternal rank. American Zoologist. 1991;31:306–317. [Google Scholar]
- Honess PE, Johnson PJ, Wolfensohn SE. A study of behavioural responses of non-human primates to air transport and re-housing. Laboratory Animals. 2004;38:119–132. doi: 10.1258/002367704322968795. [DOI] [PubMed] [Google Scholar]
- Horrocks J, Hunte W. Maternal rank and offspring rank in vervet monkeys: an appraisal of the mechanisms of rank acquisition. Animal Behaviour. 1983;31(3):772–782. [Google Scholar]
- Jenks SM, Weldele ML, Frank LG, Glickman SE. Acquisition of matrilineal rank in captive spotted hyaenas: emergence of a natural social system in peer-reared animals and their offspring. Animal Behaviour. 1995;50:893–904. [Google Scholar]
- Judge P, Griffaton N, Fincke A. Conflict management by hamadryas baboons (Papio hamadryas hamadryas) during crowding: a tension-reduction strategy. American Journal of Primatology. 2006;68(10):993–1006. doi: 10.1002/ajp.20290. [DOI] [PubMed] [Google Scholar]
- Kawai M. On the rank system in a natural group of Japanese monkeys, the basic and the dependent rank. Primates. 1958;1:111–130. (in Japanese) [Google Scholar]
- Kohn JN, Snyder-Mackler N, Barreiro LB, Johnson ZP, Tung J, Wilson ME. Dominance rank causally affects personality and glucocorticoid regulation in female rhesus macaques. Psychoneuroendocrinology. 2016;74:179–188. doi: 10.1016/j.psyneuen.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivula K, Lahti K, Orell M, Rytkönen S. Prior residency as a key determinant of social dominance in the willow tit (Parus montanus) Behavioral Ecology and Sociobiology. 1993;33(4):283–287. [Google Scholar]
- Kulik L, Amici F, Langos D, Widdig A. Sex differences in the development of aggressive behavior in rhesus macaque (Macaca mulatta) International Journal of Primatology. 2015;36:764–789. doi: 10.1007/s10764-015-9826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFreniere PJ, Charlesworth WR. Effects of friendship and dominance status on preschooler’s resource utilization in a cooperative/competitive paradigm. International Journal of Behavioral Development. 1987;10:345–358. [Google Scholar]
- Loy J, Loy K. Behavior of an all-juvenile group of rhesus monkeys. American Journal of Physical Anthropology. 1974;40:83–96. doi: 10.1002/ajpa.1330400109. [DOI] [PubMed] [Google Scholar]
- Maccoby EE, Jacklin JN. Sex differences in aggression: a rejoinder and reprise. Child Development. 1980;51:964–980. [PubMed] [Google Scholar]
- Mascaro O, Csibra G. Representation of stable social dominance relations by human infants. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(18):6862–6867. doi: 10.1073/pnas.1113194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Anxiolytic effects of the GABAA receptor partial agonist, L-838, 417: Impact of age, test context familiarity, and stress. Pharmacology Biochemistry and Behavior. 2013;109:31–37. doi: 10.1016/j.pbb.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C, Duboscq J, Dubuc C, Ginting A, Maulana Irwan A, Agil M, Widdig A, Engelhardt A. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Animal Behaviour. 2011;82:911–921. [Google Scholar]
- Nezlek JB. Reactions to daily events as a function of familiarity with an environment. European Journal of Personality. 2007;21(6):811–822. [Google Scholar]
- Norikoshi K. The development of peer-mate relationships in Japanese macaque infants. Primates. 1974;15(1):39–46. [Google Scholar]
- Novak M, Kinsey J, Jorgensen M, Hazen T. Effects of puzzle feeders on pathological behavior in individually housed rhesus monkeys. American Journal of Primatology. 1998;46(3):213–27. doi: 10.1002/(SICI)1098-2345(1998)46:3<213::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Paquette D. Fighting and playfighting in captive adolescent chimpanzees. Aggressive Behavior. 1994;20:49–65. [Google Scholar]
- Pellegrini AD, Roseth CJ, Mliner S, Bohn CM, Van Ryzin M, Vance N, Cheatham CL, Tarullo A. Social dominance in preschool classrooms. Journal of Comparative Psychology. 2007;121(1):54–64. doi: 10.1037/0735-7036.121.1.54. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. On knowing it’s only play: the role of play signals in play fighting. Aggression and Violent Behavior. 1996;1:249–268. [Google Scholar]
- Perry S, Godoy I, Lammers W, Lin A. Impact of personality traits and early life experience on timing of emigration and rise to alpha male status for wild male white-faced capuchin monkeys (Cebus capucinus) at Lomas Barbudal Biological Reserve, Costa Rica. Behaviour. 2017;154(2):195–226. [Google Scholar]
- Pun A, Birch SAJ, Baron AS. Infants use relative numerical group size to infer social dominance. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(9):2376–2381. doi: 10.1073/pnas.1514879113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun A, Birch SAJ, Baron AS. Foundations of reasoning about social dominance. Child Development Perspectives. 2017;0(0):1–6. doi: 10.1111/cdep.12235. [DOI] [Google Scholar]
- Pusey AE, Oehlert GW, Williams JM, Goodall J. Influence of ecological and social factors on body mass of wild chimpanzees. International Journal of Primatology. 2005;26(1):3–31. [Google Scholar]
- Ross HS, Conant CL, Cheyne JA, Alevizos E. Relationships and alliances in the social interaction of kibbutz toddlers. Social Development. 1992;1(1):1–17. [Google Scholar]
- Russon AE, Waite BE, Rochester MJ. Direct caregiver intervention in infant peer social interactions. American Journal of Orthopsychiatry. 1990;60(3):428–439. doi: 10.1037/h0079157. [DOI] [PubMed] [Google Scholar]
- Sandel AA, Reddy RB, Mitani JC. Adolescent male chimpanzees do not form a dominance hierarchy with their peers. Primates. 2017;58(1):39–49. doi: 10.1007/s10329-016-0553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin-Williams RC. Dominance in a human adolescent group. Animal Behaviour. 1977;25(2):400–406. doi: 10.1016/0003-3472(77)90014-8. [DOI] [PubMed] [Google Scholar]
- Schino G, Scucchi S, Maestripieri D, Turillazzi PG. Allogrooming as a tension-reduction mechanism: a behavioral approach. American Journal of Primatology. 1988;16(1):43–50. doi: 10.1002/ajp.1350160106. [DOI] [PubMed] [Google Scholar]
- Sharpe LL. Play fighting does not affect subsequent fighting success in wild meerkats. Animal Behaviour. 2005;69(5):1023–1029. [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Patterns of coalition formation by adult female baboons in Ambolseli, Kenya. Animal Behaviour. 2004;67:573–582. [Google Scholar]
- Small MF. Social climber: independent rise in rank by a female barbary macaque (Macaca sylvanus) Folia Primatologica. 1990;55:85–91. doi: 10.1159/000156503. [DOI] [PubMed] [Google Scholar]
- Smith PK, Boulton M. Rough-and-tumble play, aggression, and dominance perception and behaviour in children’s encounters. Human Development. 1990;33:271–282. [Google Scholar]
- Smith JE, Van Horn RC, Powning KS, Cole AR, Graham KE, Memenis SK, Holekamp KE. Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behavioral Ecology. 2010;21(2):284–303. [Google Scholar]
- Snyder-Mackler N, Kohn JN, Barreiro LB, Johnson ZP, Wilson ME, Tung J. Social status drives social relationships in groups of unrelated female rhesus macaques. Animal Behaviour. 2016;111:307–317. doi: 10.1016/j.anbehav.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stayer FF, Strayer J. An ethological analysis of social agonism and dominance relationships among preschool children. Child Development. 1976;47(4):980–989. [Google Scholar]
- Strayer FF, Trudel M. Developmental changes in the nature and function of social dominance among young children. Ethology and Sociobiology. 1984;5(4):279–295. [Google Scholar]
- Strayer FF, Noel JM. In: The prosocial and antisocial functions of preschool aggression: an ethological study of triadic conflict among young children. Zahn-Waxler C, Cummings M, Iannotti R, editors. Cambridge: Cambridge University Press; 1986. Altruism and aggression (107–131) [Google Scholar]
- Suomi SJ. Early determinants of behaviour: evidence from primate studies. British Medical Bulletin. 1997;53(1):170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Thierry B. Unity in diversity: lessons from macaque societies. Evolutionary Anthropology. 2007;16:224–238. [Google Scholar]
- Thomsen L, Frankenhuis WE, Ingold-Smith M, Carey S. Big and mighty: preverbal infants mentally represent social dominance. Science. 2011;331(6016):477–480. doi: 10.1126/science.1199198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behaviour of adolescent and adult rats: role of familiarity of the test situation. Alcoholism Clinical & Experimental Research. 2002;26(10):1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Wattanakulab W, Edwardsa SA, Stewarta AH, Englishb PR. Effect of familiarity with the environment on the behaviour and performance response of sows and piglets to grouping during lactation. Applied Animal Behaviour Science. 1998;61(1):25–39. [Google Scholar]
- Wilson JH. Prolactin in rats is attenuated by conspecific touch in a novel environment. Cognitive, Affective, & Behavioral Neuroscience. 2001;1(2):199–205. doi: 10.3758/cabn.1.2.199. [DOI] [PubMed] [Google Scholar]
- Wooddell LJ, Kaburu SSK, Rosenberg KL, Meyer JS, Suomi SJ, Dettmer AM. Correction: Matrilineal behavioral and physiological changes following the removal of a non-alpha matriarch in rhesus macaques (Macaca mulatta) PLoS ONE. 2016;11(6):e0157108. doi: 10.1371/journal.pone.0157108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooddell LJ, Kaburu SSK, Suomi SJ, Dettmer AM. Differential rank acquisition in semi-free ranging male and female juvenile rhesus macaques (Macaca mulatta) Proceedings of the Joint Meeting of the American Society of Primatologists and International Primatological Society. 2016 Abstract #7060. [Google Scholar]
- Wooddell LJ, Kaburu SSK, Suomi SJ, Dettmer AM. Elo-rating for tracking rank fluctuations following demographic changes involving semi-free ranging rhesus macaques (Macaca mulatta) Journal of the American Assocation for Laboratory Animal Science. 2017;56(3):1–9. [PMC free article] [PubMed] [Google Scholar]
- Zuur AF, Hilbe J, Ieno EN. A Beginner’s Guide to GLM and GLMM with R: A Frequentist and Bayesian Perspective for Ecologists. Highland Statistics; 2013. [Google Scholar]