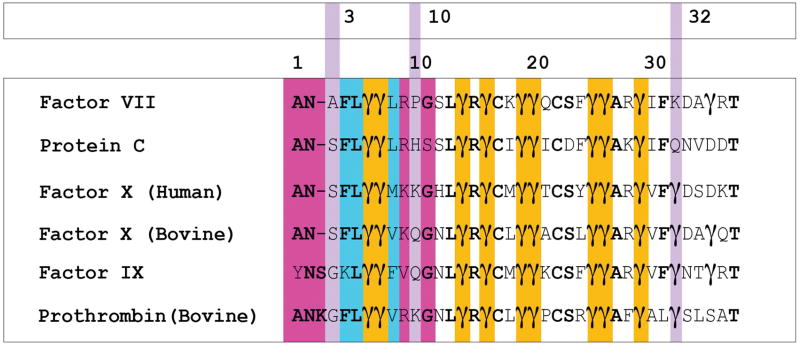
Figure 7.
The BLAST sequence alignment of coagulation factor GLA domains discussed in this study, namely: phosphatidic-acid (PA) specific domains factor VII and protein C; and PS specific domains factor X (human), factor X (bovine), factor IX, prothrombin (bovine). Unless specified otherwise, all sequences are human in origin. Cyan indicates residues of the keel (residues 4, 5, and 8). Gold indicates GLA-residues whose positions are conserved among all GLA domains. Violet is used to indicate location of residues involved in membrane interactions which differ between FX-GLA and FVII-GLA, and the FX-GLA sequence number for these residues is reported in the top row of numbers. At sequence position 3, SER3 in FX-GLA is replaced by ALA3 in FVII-GLA. At sequence position 10, which was found to participate interactions with lipid phosphate groups, the PA-specific domains carry hydrophobic residues, while the PS-specific domains contain residues capable of interactions with anionic lipids. At sequence position 32, PA-specific domains contain positively charged residues, while the PS-specific domains contain negatively charged residues. The bottom row of numbers marks every 10th sequence position in the alignment. Magenta is used to highlight residues in the ω-loop, residues 1–11, which were not otherwise highlighted.