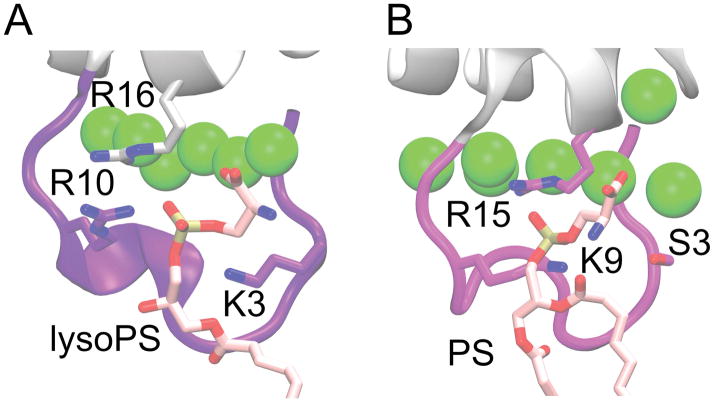
Figure 8.
Comparison of phosphate binding site for A) prothrombin-GLA co-crystallized with a single lysoPS (PDB 1NL2) [8], and B) FX-GLA bound to PS from one of our full-membrane simulations. Binding of PS is similar in both cases, with the equivalent starboard face residues (ARG10 and ARG16 on prothrombin-GLA, LYS9 and ARG15 on FX-GLA) interacting with the phosphate group. SER3 in FX-GLA does not interact with the phosphate, however, unlike LYS3 in the prothrombin structure.