Abstract
Approximately 20% of high-grade serous ovarian cancers are homologous-recombination (HR)-deficient due to genetic and epigenetic mutations of HR pathway genes including the tumor suppressor genes BRCA1 and 2. HR deficiency (HRD) compromises cells’ ability to efficiently repair DNA damage, but it also increases sensitivity to chemotherapeutic treatment strategies; however, not all ovarian cancer patients with HRD tumors exhibit positive responses to chemotherapy. Our previous iTRAQ-based comprehensive proteomic characterization of high-grade serous ovarian carcinomas found that lower levels of histone H4 acetylation at Lys12 and Lys16 (H4-K12acK16ac) were associated with HRD tumors compared with non-HRD tumors. In the current study, we developed and validated an H4-K12acK16ac parallel-reaction-monitoring (PRM)-targeted mass-spectrometry-based assay to analyze acetylation changes of histone H4 and to determine the association of these changes with total H4, histone acetyltransferase, and histone deacetylase (HDAC) levels. Whereas the levels of H4 and histone acetyltransferases were stable irrespective of HRD status, the levels of histone H4 acetylation and one HDAC, HDAC6, were elevated in the HRD tumors. Relative H4 acetylation levels were also analyzed by an antibody-based approach in additional ovarian tumors. It is possible that specific H4 acetylation at Lys12 and Lys16 associated with HRD could inform chemotherapeutic treatment modalities to improve ovarian cancer patients’ treatment response.
Keywords: parallel reaction monitoring (PRM), histone, lysine acetylation, ovarian cancer, homologous recombination, targeted assays, quantification, mass spectrometry
Graphical Abstract

INTRODUCTION
Epithelial ovarian cancer is the most lethal gynecological malignancy in the United States, and the 5-year survival rate of patients with advanced stage ovarian cancer is only 26.9%.1 A significant challenge in chemotherapeutic treatment regimens for ovarian cancer patients is that the response to platinum-based chemotherapy is often very poor because of the development of chemoresistance, and thus novel treatment strategies are needed.
A recent advance in translational oncology research is that the mutational status of a solid tumor can predict the therapeutic efficacy for a specific drug in a molecularly defined subset of patients.2,3 Genetic variants in genes involved in the distribution, metabolism, accumulation, or repair of lesions can influence the response of drugs that are used in the treatment of ovarian cancer.4 An impactful example of the study of the genomic prediction of therapeutic efficacy is that of inhibitors of poly(ADP-ribose) polymerase (PARPi) that have emerged as a novel class of anticancer drugs to treat homologous recombination deficiency (HRD)-related ovarian cancer associated with loss of BRCA1/2 function due to mutations or the down-regulation of the expression of BRCA1/2 and associated genes.5 PARP inhibitors interrupt the DNA repair process by impairing two mechanisms of PARP: (1) blocking binding sites of single-strand breaks (SSBs) with its own zinc finger domains, consequently directly blocking DNA access by PARP, and (2) preventing the transfer of ADP-ribose to form PAR chains to block the formation of the base excision repair complex.6–8
Because of the therapeutic advantages they confer in HRD tumors, many PARP inhibitors are in various stages of clinical trials. However, HRD can be manifested in several ways other than genomic BRCA1/2 mutational analysis.9–12 A vital key to the success of ovarian cancer treatment strategies based on the genomic prediction of therapeutic efficacy, such as PARPi treatment, is a more thorough understanding of ovarian cancer etiology. Although there has been extensive genomic and transcriptomic characterization of ovarian cancer aimed at defining the genomic landscape and assisting the development of targeted therapies,13,14 less is known about how the ovarian cancer genome drives the cancer proteome with effects on clinical outcomes. Thus there is a great need for these types of studies given that functions encoded in the genome are executed at the protein level with the potential for further modulation by post-translational modifications (PTMs).15
Lysine acetylation is a reversible PTM that is controlled by the opposing activities of lysine acetyltransferases and deacetylases.16 The dysregulation of histone acetylation has been implicated in various human diseases including cancer, and thus histone deacetylases (HDACs) are attractive anticancer therapeutic targets.17 Previously, we conducted an isobaric tags for relative and absolute quantitation (iTRAQ)-based comprehensive proteomic characterization of high-grade serous ovarian carcinomas toward the determination of the underlying molecular mechanisms that are associated with HRD in ovarian cancer to identify putative biomarkers that could be used to stratify ovarian cancer patients for treatment.18 One of our main observations was that lower levels of histone H4 acetylation at Lys12 and Lys16 (H4-K12acK16ac) correlated with HRD status. Indeed, the loss of H4 monoacetylation in cancer cells has been demonstrated to occur predominantly at Lys16.19 Our iTRAQ data indicating lower H4-K12acK16ac levels in HRD tumors was supported by the results from a sequential window acquisition of all theoretical mass spectra (SWATH-MS) analysis of the same tumors.18
Parallel reaction monitoring (PRM) is a targeted proteomics strategy where all product ions of the target peptides are simultaneously monitored at high resolution and high mass accuracy, thus enabling high-quality quantitative measurements.20,21 The use of PRM to quantify changes in histone modifications was first introduced by Tang et al. in 2014.22 In the current study, we developed, validated, and deployed a targeted mass-spectrometry-based H4-K12acK16ac PRM assay to verify our initial proteomic findings, demonstrating that lower H4-K12acK16ac levels are associated with HRD status in ovarian tumors. The results from the H4-K12acK16ac PRM assay were further verified by applying the PRM assay and Western blot to additional ovarian tumors. We also compared the expression of acetylated H4 to the total H4 protein level as well as that of histone acetyltransferases and histone deacetylases (HDACs), and we observed an HRD-associated level of HDAC6 overexpression, which supports a potential role for the effectiveness of HDAC inhibitor treatment in HRD tumors that are characterized by low levels of H4 lysine acetylation.
EXPERIMENTAL SECTION
Materials
Tris(2-carboxyethyl)phosphine (TCEP), urea, LC-MS grade water, and LC-MS grade acetonitrile were acquired from Thermo Fisher Scientific. Sodium citrate, Tween-20, iodoacetamide, ammonium bicarbonate, and formic acid were obtained from Sigma-Aldrich. Sequencing-grade trypsin was obtained from Promega. All chemicals were of analytical grade unless otherwise specified.
Clinical Specimens
The TCGA frozen ovarian tumor tissue specimens were obtained through the CPTAC Biospecimen Core Resource, as previously described.18 The BRCA mutant (n = 4) and WT (n = 3) ovarian tumor tissues were obtained from Dr. Ie-Ming Shih from the Department of Gynecology and Obstetrics, Johns Hopkins Medical Institutions and Dr. Douglas Levine from the Department of Gynecologic Oncology, Laura and Isaac Perlmutter Cancer Center, New York University Langone Medical Center, according to IRB-approved protocols.
Western Blotting
Ten μg protein from the BRCA WT (n = 3) and mutant (n = 4) ovarian tumor tissue homogenized in urea buffer (8 M urea, 0.8 M NH4HCO3, pH 8.0) was separated on 4–12% Bis-Tris gels (Thermo Fisher Scientific), followed by electrophoretic transfer to nitrocellulose membranes. Nonspecific binding sites on the blot were blocked with 5% milk/TBS-T, followed by overnight incubation at 4 °C in primary antibody (rabbit anti-Histone H4 acetyl K16, 1:1500, Abcam; rabbit anti-histone H4 acetyl K12, 1:10 000, Abcam; rabbit anti-Histone H4, 1:1000, Abcam; rabbit anti-actin-HRP, 1:10:000, Sigma). Goat anti-rabbit secondary antibody was used at a 1:10 000 dilution. Immunoreactive bands were visualized using chemiluminescence (SuperSignal West Pico Chemiluminescent Substrate, Thermo Fisher Scientific). Densitometric analysis was conducted using ImageJ (NIH).
Protein Extraction and Tryptic Digestion
Approximately 50 mg of each of the TCGA ovarian tumor tissues was sonicated in 1.5 mL of 8 M urea, 0.8 M NH4HCO3, pH 8.0. Proteins were reduced with 10 mM TCEP for 1 h at 37 °C and subsequently alkylated with 12 mM iodoacetamide for 1 h at RT in the dark. Samples were diluted 1:4 with deionized water and digested with trypsin at a 1:50 enzyme-to-protein ratio. After 12 h of digestion at 37 °C, another aliquot of the same amount of trypsin was added to the samples and further incubated at 37 °C overnight. The digested samples were then acidified, cleaned up (SCX and C18), and dried in a vacuum centrifuge. The FFPE ovarian tissue sections were processed and subjected to trypsin digestion according to the procedure described in Tian et al.23
Parallel Reaction Monitoring Analysis
Tier 2 assay development was conducted using crude light and heavy stable isotope-labeled peptide standards (SIS; ~60% chemical purity, >99% isotopic purity, Thermo Fisher Scientific PEPotec SRM peptide library). Histone H4-K12acK16ac peptide sequence: 9GLGKacGGAKacR17. The SIS peptide incorporated a fully atom-labeled 13C and 15N isotope at the C-terminal arginine (R). Peptides were provided in 0.1% TFA/ 50% ACN and stored at −80 °C until use.
PRM analysis was conducted using a Dionex UltiMate 3000 RSLCnano LC system (Thermo Fisher Scientific) coupled to a Q-Exactive mass spectrometer (Thermo Fisher Scientific). The peptides were injected (6 μL) onto a C18 trap column (300 μm I.D. × 5 mm packed with Acclaim PepMap 100, 5 μm, 100 Å C18; Thermo Fisher Scientific) at a loading pump flow rate of 5 μL/min, followed by separation on a 75 μm I.D. × 50 cm EASY-Spray analytical column packed with 2 μm Acclaim PepMap RSLC C18 (Thermo Fisher Scientific). Mobile phase A was 2% ACN/0.1% formic acid in water, and mobile phase B was 90% ACN/0.1% formic acid. The column was heated to 42 °C. Separations were performed at 250 nL/min across a 20 min linear gradient from 4–40%B.
An EASY-Spray source (Thermo Fisher Scientific) with zero dead volume nanoViper fittings was used with the Q-Exactive. The spray voltage was 1.8 kV and the capillary temperature was 250 °C. The mass spectrometer was operated in a targeted-MS2 acquisition mode with a maximum IT of 100 ms, 1 microscan, 70 000 resolution, 5e5 AGC target, 2.0 m/z isolation window, and 29% normalized collision energy. Intrarun mass calibration was conducted using lock masses of 445.12003 m/z and 371.10123 m/z. A scheduled PRM method was created to monitor the 2+ charge state of each peptide with a 5 min retention time window. For the PRM analysis of the ovarian tumor specimens, each injection consisted of 1 μg of peptides from each specimen spiked with 200 fmol of the heavy H4-K12acK16ac peptide.
PRM Assay Characterization: Response Curve
Response (calibration) curves were generated using light peptides spiked into and serially diluted with a biological matrix consisting of tryptic peptides derived from digested human ovarian tumor tissue. The heavy peptides were spiked in at a constant concentration of 33.3 fmol/μL (200 fmol on column) to enable normalization. Peak area ratios (light/heavy) were used as the dependent variables to generate the response curves. The seven-point response curves covered four orders of magnitude in abundance range, and they were run in triplicate in order of increasing concentration with three blank runs prior to the first replicate run of the curve and two blank runs following each curve. The assays were characterized based on several metrics including LOD, LLOQ, and linearity.
PRM Assay Characterization: Reproducibility
Assay performance reproducibility (intraday, interday, and total assay CV) was measured across 5 days in triplicate at three levels, low, medium, and high, to approximate 2× LLOQ, 50× LLOQ, and 100× LLOQ, respectively. The total assay CV was calculated as the square root of the sum of squares of the intra and interday CVs. The run order was randomized to more accurately reflect the variability in assay performance.
Data and Statistical Analysis
The raw PRM data were processed using Skyline.24 All assay details, assay parameters, response curves, repeatability data, detailed standard operating protocols, and additional assay-specific resources can be located on the CPTAC Assay Portal https://assays.cancer.gov/ using the search term “CPTAC-1055”. Links to the assay development data on Panorama are available through the CPTAC-1055 assay page on the Assay Portal. Exported Skyline data from the PRM analysis of the ovarian tumor tissue specimens were analyzed by a Mann-Whitney U-test to determine the statistical significance of the peak area ratios. p-values <0.05 were considered significant.
RESULTS AND DISCUSSION
Histone H4-K12acK16ac PRM Assay Development
To verify our previous observation that lower levels of H4-K12acK16ac are directly correlated with HRD status in high-grade serous ovarian tumors, we developed and validated a targeted mass spectrometry-based assay to measure the relative abundance of H4-K12acK16ac. Crude light and heavy H4-K12acK16ac peptides (H4 amino acids 9–17) were synthesized, and a PRM assay was developed as a Tier 2 assay according to the framework for targeted MS-based assay “fit-for-purpose” validation established by the National Cancer Institute Clinical Proteomic Tumor Analysis Consortium (NCI CPTAC).25–28 By definition, Tier 2 assays have a moderate-to-high degree of analytical validation, include labeled internal standards for every analyte, and have high specificity, moderate to high precision (20–35% CV), and high repeatability.
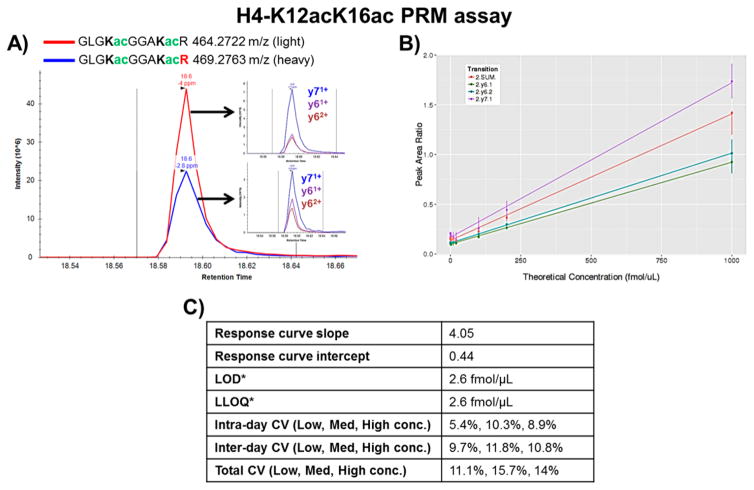
To ensure the specificity of the PRM assay, all of the transitions from the light and heavy H4-K12acK16ac peptides that were selected to be monitored (y71+, y61+, and y62+) included both of the modified lysine residues (K12 and K16, Figure 1A). After establishing the scheduled method using these three transitions, the assay was validated by running a response curve in triplicate that covered four orders of magnitude (1 fmol/μL to 1 pmol/μL, Figure 1B). On the basis of the response curve data, the estimated limit of detection (LOD) and lower limit of quantification (LLOQ) of the assay were determined to be ~2.6 fmol/μL (Figure 1C). LOD, the lowest analyte concentration at which the signal is distinguishable from the noise, was calculated based on the average signal from three blank injections + 3* standard deviation of the peak area ratios observed for the lowest concentration. LLOQ, the lowest concentration of the analyte at which quantitative measurements can be made, was determined to be the lowest concentration at which the CV was <20%. It should be noted that because unpurified synthetic peptides were used to develop and validate the assay, the precise amount of each peptide standard was unknown. (Approximate amounts based on the values reported by the peptide vendor were used for all subsequent calculations.) Accordingly, the reported values for LOD and LLOQ are not absolute values.
Figure 1.
Validation of H4-K12acK16ac PRM assay. (A) Representative XICs of the light and heavy peptides. Insets show XICs of the transitions for each peptide. (B) Response curve. The peak area ratio (light/heavy) for each transition is plotted versus the theoretical concentration (fmol/μL). The curve was run in triplicate. (C) Assay validation parameters determined from the response curve and the 5 day repeatability experiment. *Concentrations are approximate as the assay was developed and validated using crude peptides (~60% purity).
To determine the performance of the H4-K12acK16ac assay across several days, a mini-validation of repeatability experiment was conducted wherein the assay was run at three concentration levels (low, medium, high) in triplicate on 5 days. The assay performance was assessed based on the intraday, interday, and total CVs (Figure 1C). In agreement with the acceptable performance criteria for Tier 2 assays, the Total CVs of the assay at the low, medium, and high concentration levels were all <20%. All assay resources are located on the CPTAC Assay Portal https://assays.cancer.gov/ (assay no. CPTAC-1055).
Verification of Low H4-K12acK16ac Levels in HRD versus non-HRD Ovarian Tumors
After the development and validation of the Tier 2 H4-K12acK16ac PRM assay, we used the assay to assess the abundance of H4-K12acK16ac in the subset of the TCGA high-grade serous ovarian tumors that we analyzed by iTRAQ- and SWATH-based proteomics.18 Of the initial total of 122 TCGA high-grade serous ovarian tumors to which we had access, only 97 were analyzed by PRM due to the availability of tumors containing a sufficient amount of peptides remaining after other proteomic analyses including iTRAQ and SWATH. Among the 97 ovarian tumor tissue specimens analyzed by PRM, 80 tumors (45 HRD, 35 non-HRD) were detected with H4-K12acK16ac signals above the LLOQ. The median relative abundance of H4-K12acK16ac (determined by the PRM peak area ratio of the endogenous light/synthetic spiked-in heavy peptide) in the non-HRD tumors was 1.34-fold higher than the HRD tumors, and the overall difference in the level of H4-K12acK16ac between the HRD and non-HRD tumors was significant (p = 0.029, Mann-Whitney U-test; Figure 2A, Supplemental Table 1). These results verify our previous observation that lower H4-K12acK16ac levels are associated with HRD status in ovarian tumors.
Figure 2.
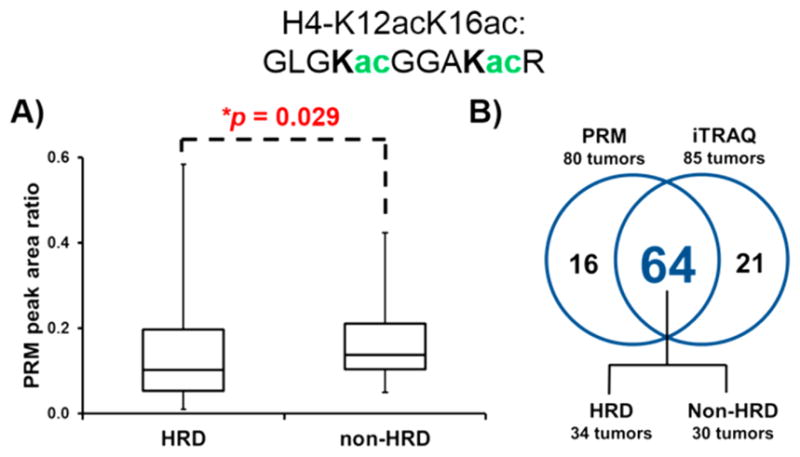
Lower relative abundance of H4-K12acK16ac in HRD versus non-HRD tumors. (A) H4-K12acK16ac was measured by PRM in 45 HRD and 35 non-HRD tumors. (B) Quantifiable levels of H4-K12acK16ac were detected in 64 (34 HRD and 30 non-HRD) of the tumors that were analyzed by iTRAQ and PRM.
With the exception of one tumor, the relative abundance of H4-K12acK16ac in all of the HRD tumors was within two standard deviations of the mean. The relative abundance of H4-K12acK16ac in one of the HRD tumors exceeded four standard deviations of the mean, and thus it could be considered as an outlier. The relatively high level of H4-K12acK16ac in this tumor could be indicative of the intratumor heterogeneity of the specimen, wherein the H4-K12acK16ac levels could vary according to cell type. Omitting this tumor from the panel of HRD tumors increases the significance of the H4-K12acK16ac level between the HRD and non-HRD tumors to p = 0.017, and the magnitude of the median relative abundance of H4-K12acK16ac in the non-HRD tumors compared with the HRD tumors increases to 1.41-fold. A systematic analysis of the purity of the TCGA ovarian tumors indicated that this specimen had an intermediate Consensus Measurement of Purity Estimator (CPE) score of 0.798 (on a scale of 0 to 1.0, with a score of 1.0 indicating the highest level of purity).29 However, it should be noted that tumor purity was not considered as a covariate in the present study, and thus conclusive statements cannot be made regarding any potential causal associations between tumor purity and H4-K12acK16ac levels.
Reflecting the increased sensitivity of PRM as a targeted MS method compared with an untargeted method such as iTRAQ, H4-K12acK16ac was only detected in 70% of the tumors that were analyzed via iTRAQ (85/122) compared with 82% of the tumors that were analyzed via PRM (80/97). There were 64 tumors (34 HRD, 30 non-HRD) in which H4-K12acK16ac was detected by both iTRAQ and PRM (Figure 2B). Of the 21 tumors in which H4-K12acK16ac was exclusively detected by iTRAQ, 6 were also analyzed by PRM and had undetectable H4-K12acK16ac levels. The unique detection of H4-K12acK16ac in 16 tumors by PRM and 21 tumors by iTRAQ is likely indicative of the fundamental differences in targeted proteomics strategies compared with shotgun proteomics approaches with one obvious difference that our targeted proteomics approach entailed 1D online LC-MS/MS and the shotgun proteomics approach used 2D LC-MS/MS with offline basic reverse-phase fractionation, followed by online LC-MS/MS.30–34
To compare the iTRAQ versus PRM quantification of H4-K12acK16ac, the iTRAQ ratios (signal intensity of the H4-K12acK16ac in an individual sample normalized to a common control sample) and the PRM peak area ratios (endogenous light-peptide XIC peak area normalized to the spiked-in heavy-peptide XIC peak area) were converted to z-scores, and the Pearson correlation coefficient (r) was 0.35 (p < 0.005), indicating a positive correlation between the iTRAQ- and PRM-based measurements of H4-K12acK16ac despite the fundamental differences in the respective methods of quantification (Supplemental Table 2). Additional statistical analysis was performed to further analyze the similarity between the PRM and iTRAQ data. A p value of 0.60 was obtained when conducting a Wilcoxon signed-rank test for paired samples to determine whether the true median difference between the PRM and iTRAQ measurements of the samples was 0. Thus this p value indicates that the null hypothesis of a median difference of 0 between the paired PRM and iTRAQ measurements of the same samples cannot be rejected.
To further confirm the results from our proteomic studies, we conducted Western blot analysis of Histone H4 acetylation in additional BRCA WT (n = 3) and BRCA mutant (n = 4) ovarian tumors. The loss of H4 monoacetylation in human tumor cells has been demonstrated to occur predominantly at K16 prior to K12, and the loss of H4 acetylation at K16 is a common hallmark of cancer.19 Our results indicated lower H4-K16ac expression levels in the BRCA mutant compared with the WT tumors when normalized to total H4 levels (p = 0.034), whereas the levels of H4-K12ac normalized to total H4 levels were unchanged (Figure 3A). Neither of these monoacetylated H4 peptides were detected in the TCGA ovarian tumors using an iTRAQ-based global proteomic approach, suggesting that the diacetylated H4-K12acK16ac peptide could have a higher level of relative abundance compared with the monoacetylated H4 peptides.
Figure 3.
Detection of H4-K12ac and H4-K16ac in an independent set of WT and mutant BRCA ovarian tumor tissues. (A) Western blot analysis of H4-K12ac, H4-K16ac, total H4, and actin expression. Densitometric analysis (lower panel). (B) PRM analysis of the H4-K12acK16ac expression in the same tissues. n.s., not significant.
We also applied our developed H4-K12acK16ac PRM assay to the additional BRCA WT (n = 3) and mutant (n = 4) ovarian tumor specimens, and the average relative abundance of H4-K12acK16ac was approximately two times higher in the BRCA WT versus the mutant tumors (Figure 3B). However, the difference between the relative abundance levels of H4-K12acK16ac in the BRCA WT and mutant tumors was not significant. The lack of a significant difference in the H4-K12acK16ac levels could be reflective of the relatively small sample size (n = 7) compared with that of the TCGA tumor specimens (n = 97).
Decreased H4 Acetylation in HRD Tumors Is Associated with Elevated HDAC6 Levels
To determine whether the decreased H4 acetylation in HRD tumors is regulated at the total protein level or at the post-translational level, it is important to quantify not only the modified form of the peptide(s) of interest but also the corresponding unmodified form of the peptide(s) and the total protein.35 However, in the case of the H4-K12acK16ac peptide, the corresponding unmodified peptide cannot be readily detected by a standard shotgun proteomics sample preparation workflow using trypsin as the cleavage enzyme. The lack of an acetyl group on unmodified K12 and K16 enables trypsin to cleave at those sites, consequently generating three peptides with low masses: 9GLGK12: 187.73 m/z (2+); 13GGAK16: 166.69 m/z (2+); and R17:175.12 m/z (1+). These masses are below the lower full MS1 scan range cutoff of 400 m/z that was used to acquire the iTRAQ data for the TCGA high-grade serous ovarian tumors.18 Additionally, the mass-spectrometric method that was used to acquire the iTRAQ data included precursor ion charge-state screening wherein singly charged ions were rejected to enable the preferred selection of ions with z ≥ 2, which are predominantly tryptic peptides.
The quantification of the total H4 protein level in our global proteomic iTRAQ data set (33 unique peptides) indicated the lack of a significant difference in the relative abundance of this protein based on the HRD status of the ovarian tumors (Supplemental Table 3). Statistical analysis of the total H4 protein-normalized H4-K12acK16ac levels indicated significantly lower levels in the HRD versus non-HRD tumors (p = 0.015, Mann-Whitney U-test). This observation supports our hypothesis that it is the post-translational acetylation modification of H4 and not the level of the unmodified protein that is associated with HRD status.
We also analyzed the global levels of the histone acetyltransferases (EP300, KAT2A, KAT5, KAT6A, KAT7, and KAT8) and deacetylases (HDAC1-10) with known roles in H4 acetylation36,37 regardless of whether they were detected across all of the 122 TCGA ovarian tumor specimens (Supplemental Table 3). The relative abundance levels of the acetyltransferases did not differ significantly between the HRD and non-HRD tumors. Interestingly, the level of hMOF/KAT8, an important histone acetyltransferase that is responsible for the basal level of H4-K16 acetylation,38 was unchanged. This could suggest a more influential role of HDACs in the difference between H4-K16 acetylation levels in high-grade serous ovarian tumors.
Whereas there was a lack of an HRD-associated difference in acetyltransferase expression, we observed elevated levels of HDAC6 expression in the HRD versus non-HRD tumors (Figure 4). Among the 10 HDACs that were quantified by iTRAQ, only HDAC6 had significantly increased expression in the HRD tumors (p = 0.020, Mann-Whitney U-test). HDAC6 overexpression is known to cause resistance to chemo-therapeutic drugs, and it is also associated with poor prognosis in lung adenocarcinoma.39 Analysis of the association between total H4 protein-normalized H4-K12acK16ac levels and HDAC6 abundance indicated lower levels in the HRD versus non-HRD tumors (p = 0.018, Mann-Whitney U-test), thus providing additional evidence of an association between decreased H4-K12acK16ac levels and increased HDAC6 levels in the HRD tumors. In a separate study using a pathway-based differential dependency network approach to assess HRD-associated differences in acetylation-related proteins,40 we previously showed that the levels of HDAC1 and associated proteins were increased at the pathway level together with decreased acetylation of H4 in HRD tumors at the PTM level.18
Figure 4.

HRD-associated elevated expression of HDAC6. Among the 10 HDACs that were quantified by iTRAQ, only HDAC6 had significantly increased expression in the HRD compared with the non-HRD tumors.
CONCLUSIONS
Our previous iTRAQ-based global proteomic study of high-grade serous ovarian carcinomas indicated that the dual acetylation of H4 at Lys12 and Lys16 correlates with HRD status. This observation was verified in the current study using PRM as an orthogonal analytical mass-spectrometry-based method. Similar trends in H4 acetylation levels were confirmed by Western blot using additional ovarian tumor tissue expressing mutant or WT BRCA. Given that HRD is associated with susceptibility to PARP inhibitors and that HDAC inhibitors are attractive anti-ovarian-cancer therapeutic targets,17,41 it is possible that specific H4 acetylation events associated with HRD could inform PARPi or HDAC inhibitor chemotherapeutic treatment modalities to improve patients’ treatment response.
We have presented multiple lines of evidence that lower H4-K12acK16ac levels are significantly associated with HRD. A requisite subsequent step for the possible future assessment of the role of H4-K12acK16ac in stratifying ovarian cancer patients for PARPi treatment would be to determine whether this statistical association is valid for patients who respond to PARPi treatment. Additionally, it would be beneficial to conduct further studies toward the elucidation of the mechanism(s) that underlie the lower H4-K12acK16ac levels that are observed in ovarian cancer patients with HRD tumors.
Supplementary Material
Acknowledgments
This work was supported by funding from the National Cancer Institute Clinical Proteomic Tumor Analysis Consortium (NCI CPTAC, U24CA160036 to H.Z.).
ABBREVIATIONS
- CPTAC
Clinical Proteomic Tumor Analysis Consortium
- CV
coefficient of variation
- HRD
homologous recombination deficiency
- iTRAQ
isobaric tags for relative and absolute quantitation
- LLOQ
lower limit of quantification
- LOD
limit of detection
- PARP
poly(ADP-ribose) polymerase
- PRM
parallel reaction monitoring
- PTM
post-translational modification
- RT
retention time
- SIS
stable isotope-labeled peptide standard
- XIC
extracted ion chromatogram
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval of the final version of the manuscript.
Notes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jproteome.7b00405.
Supplemental Tables S1–S3 captions. (PDF)
Table S1: H4-K12acK16ac peak area ratios from the PRM analysis of HRD and non-HRD ovarian tumors. (XLSX)
Table S2: Z-scores of H4-K12acK16ac measured by iTRAQ and PRM in HRD and non-HRD ovarian tumors. (XLSX)
Table S3: Global protein iTRAQ ratios of H4, histone acetyltransferases and histone deacetylases (HDACs) measured in HRD and non-HRD ovarian tumors. (XLSX)
References
- 1.Kurman RJ, Shih IM. Am J Pathol. 2016;186:733. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landen CN, Jr, Birrer MJ, Sood AK. J Clin Oncol. 2008;26:995. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 3.Doroshow JH, Kummar S. Nat Rev Clin Oncol. 2014;11:649. doi: 10.1038/nrclinonc.2014.158. [DOI] [PubMed] [Google Scholar]
- 4.Caiola E, Broggini M, Marabese M. Pharmacogenomics J. 2014;14:401. doi: 10.1038/tpj.2014.32. [DOI] [PubMed] [Google Scholar]
- 5.Bell-McGuinn KM, Konner JA, Tew WP, Hensley ML, Iasonos A, Charpentier E, Mironov S, Sabbatini P, Aghajanian C. Int J Gynecol Cancer. 2016;26:255. doi: 10.1097/IGC.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ame JC, Rolli V, Schreiber V, Niedergang C, Apiou F, Decker P, Muller S, Hoger T, Murcia JM-d, de Murcia G, Menissier-de Murcia J, de Murcia G. J Biol Chem. 1999;274:17860. doi: 10.1074/jbc.274.25.17860. [DOI] [PubMed] [Google Scholar]
- 7.Helleday T. Mol Oncol. 2011;5:387. doi: 10.1016/j.molonc.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu FW, Tewari KS. Curr Treat Options Oncol. 2016;17:12. doi: 10.1007/s11864-015-0378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins JA, Irshad S, Grigoriadis A, Tutt AN. Breast Cancer Res. 2014;16:211. doi: 10.1186/bcr3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R, Bowman-Colin C, Li Y, Greene-Colozzi A, Iglehart JD, Tung N, Ryan PD, Garber JE, Silver DP, Szallasi Z, Richardson AL. Cancer Discovery. 2012;2:366. doi: 10.1158/2159-8290.CD-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulligan JM, Hill LA, Deharo S, Irwin G, Boyle D, Keating KE, Raji OY, McDyer FA, O’Brien E, Bylesjo M, Quinn JE, Lindor NM, Mullan PB, James CR, Walker SM, Kerr P, James J, Davison TS, Proutski V, Salto-Tellez M, Johnston PG, Couch FJ, Paul Harkin D, Kennedy RD. J Natl Cancer Inst. 2014;106:djt335. doi: 10.1093/jnci/djt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Telli ML, Jensen KC, Vinayak S, Kurian AW, Lipson JA, Flaherty PJ, Timms K, Abkevich V, Schackmann EA, Wapnir IL, Carlson RW, Chang PJ, Sparano JA, Head B, Goldstein LJ, Haley B, Dakhil SR, Reid JE, Hartman AR, Manola J, Ford JM. J Clin Oncol. 2015;33:1895. doi: 10.1200/JCO.2014.57.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creighton CJ, Hernandez-Herrera A, Jacobsen A, Levine DA, Mankoo P, Schultz N, Du Y, Zhang Y, Larsson E, Sheridan R, Xiao W, Spellman PT, Getz G, Wheeler DA, Perou CM, Gibbs RA, Sander C, Hayes DN, Gunaratne PH Cancer Genome Atlas Research, N. PLoS One. 2012;7:e34546. doi: 10.1371/journal.pone.0034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis MJ, Gillette M, Carr SA, Paulovich AG, Smith RD, Rodland KK, Townsend RR, Kinsinger C, Mesri M, Rodriguez H, Liebler DC Clinical Proteomic Tumor Analysis, C. Cancer Discovery. 2013;3:1108. doi: 10.1158/2159-8290.CD-13-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scholz C, Weinert BT, Wagner SA, Beli P, Miyake Y, Qi J, Jensen LJ, Streicher W, McCarthy AR, Westwood NJ, Lain S, Cox J, Matthias P, Mann M, Bradner JE, Choudhary C. Nat Biotechnol. 2015;33:415. doi: 10.1038/nbt.3130. [DOI] [PubMed] [Google Scholar]
- 17.Marks PA. Expert Opin Invest Drugs. 2010;19:1049. doi: 10.1517/13543784.2010.510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Liu T, Zhang Z, Payne SH, Zhang B, McDermott JE, Zhou JY, Petyuk VA, Chen L, Ray D, Sun S, Yang F, Chen L, Wang J, Shah P, Cha SW, Aiyetan P, Woo S, Tian Y, Gritsenko MA, Clauss TR, Choi C, Monroe ME, Thomas S, Nie S, Wu C, Moore RJ, Yu KH, Tabb DL, Fenyo D, Bafna V, Wang Y, Rodriguez H, Boja ES, Hiltke T, Rivers RC, Sokoll L, Zhu H, Shih IM, Cope L, Pandey A, Zhang B, Snyder MP, Levine DA, Smith RD, Chan DW, Rodland KD. Cell. 2016;166:755. doi: 10.1016/j.cell.2016.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, Iyer NG, Perez-Rosado A, Calvo E, Lopez JA, Cano A, Calasanz MJ, Colomer D, Piris MA, Ahn N, Imhof A, Caldas C, Jenuwein T, Esteller M. Nat Genet. 2005;37:391. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 20.Peterson AC, Russell JD, Bailey DJ, Westphall MS, Coon JJ. Mol Cell Proteomics. 2012;11:1475. doi: 10.1074/mcp.O112.020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallien S, Duriez E, Crone C, Kellmann M, Moehring T, Domon B. Mol Cell Proteomics. 2012;11:1709. doi: 10.1074/mcp.O112.019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang H, Fang H, Yin E, Brasier AR, Sowers LC, Zhang K. Anal Chem. 2014;86:5526. doi: 10.1021/ac500972x. [DOI] [PubMed] [Google Scholar]
- 23.Tian Y, Gurley K, Meany DL, Kemp CJ, Zhang H. J Proteome Res. 2009;8:1657. doi: 10.1021/pr800952h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Bioinformatics. 2010;26:966. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carr SA, Abbatiello SE, Ackermann BL, Borchers C, Domon B, Deutsch EW, Grant RP, Hoofnagle AN, Huttenhain R, Koomen JM, Liebler DC, Liu T, MacLean B, Mani DR, Mansfield E, Neubert H, Paulovich AG, Reiter L, Vitek O, Aebersold R, Anderson L, Bethem R, Blonder J, Boja E, Botelho J, Boyne M, Bradshaw RA, Burlingame AL, Chan D, Keshishian H, Kuhn E, Kinsinger C, Lee JS, Lee SW, Moritz R, Oses-Prieto J, Rifai N, Ritchie J, Rodriguez H, Srinivas PR, Townsend RR, Van Eyk J, Whiteley G, Wiita A, Weintraub S. Mol Cell Proteomics. 2014;13:907. doi: 10.1074/mcp.M113.036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoofnagle AN, Whiteaker JR, Carr SA, Kuhn E, Liu T, Massoni SA, Thomas SN, Townsend RR, Zimmerman LJ, Boja E, Chen J, Crimmins DL, Davies SR, Gao Y, Hiltke TR, Ketchum KA, Kinsinger CR, Mesri M, Meyer MR, Qian WJ, Schoenherr RM, Scott MG, Shi T, Whiteley GR, Wrobel JA, Wu C, Ackermann BL, Aebersold R, Barnidge DR, Bunk DM, Clarke N, Fishman JB, Grant RP, Kusebauch U, Kushnir MM, Lowenthal MS, Moritz RL, Neubert H, Patterson SD, Rockwood AL, Rogers J, Singh RJ, Van Eyk JE, Wong SH, Zhang S, Chan DW, Chen X, Ellis MJ, Liebler DC, Rodland KD, Rodriguez H, Smith RD, Zhang Z, Zhang H, Paulovich AG. Clin Chem. 2015;62:48. doi: 10.1373/clinchem.2015.250563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas SN, Harlan R, Chen J, Aiyetan P, Liu Y, Sokoll LJ, Aebersold R, Chan DW, Zhang H. Anal Chem. 2015;87:10830. doi: 10.1021/acs.analchem.5b02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiteaker JR, Halusa GN, Hoofnagle AN, Sharma V, MacLean B, Yan P, Wrobel JA, Kennedy J, Mani DR, Zimmerman LJ, et al. Nat Methods. 2014;11:703. doi: 10.1038/nmeth.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aran D, Sirota M, Butte AJ. Nat Commun. 2015;6:8971. doi: 10.1038/ncomms9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domon B, Aebersold R. Nat Biotechnol. 2010;28:710. doi: 10.1038/nbt.1661. [DOI] [PubMed] [Google Scholar]
- 31.Picotti P, Aebersold R. Nat Methods. 2012;9:555. doi: 10.1038/nmeth.2015. [DOI] [PubMed] [Google Scholar]
- 32.Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B. Anal Bioanal Chem. 2007;389:1017. doi: 10.1007/s00216-007-1486-6. [DOI] [PubMed] [Google Scholar]
- 33.Wenger CD, Lee MV, Hebert AS, McAlister GC, Phanstiel DH, Westphall MS, Coon JJ. Nat Methods. 2011;8:933. doi: 10.1038/nmeth.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aggarwal K, Choe LH, Lee KH. Briefings Funct Genomics Proteomics. 2006;5:112. doi: 10.1093/bfgp/ell018. [DOI] [PubMed] [Google Scholar]
- 35.Sun S, Zhang H. Anal Chem. 2015;87:6479. doi: 10.1021/acs.analchem.5b01679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang PH, Zhang L, Zhang YJ, Zhang J, Xu WF. Drug Discoveries Ther. 2013;7:233. doi: 10.5582/ddt.2013.v7.6.233. [DOI] [PubMed] [Google Scholar]
- 37.Gil J, Ramirez-Torres A, Encarnacion-Guevara S. J Proteomics. 2017;150:297. doi: 10.1016/j.jprot.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Taipale M, Rea S, Richter K, Vilar A, Lichter P, Imhof A, Akhtar A. Mol Cell Biol. 2005;25:6798. doi: 10.1128/MCB.25.15.6798-6810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Tang F, Hu P, Wang Y, Gong J, Sun S, Xie C. Oncol Rep. 2016;36:589. doi: 10.3892/or.2016.4811. [DOI] [PubMed] [Google Scholar]
- 40.Zhang B, Li H, Riggins RB, Zhan M, Xuan J, Zhang Z, Hoffman EP, Clarke R, Wang Y. Bioinformatics. 2009;25:526. doi: 10.1093/bioinformatics/btn660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newbold A, Matthews GM, Bots M, Cluse LA, Clarke CJ, Banks KM, Cullinane C, Bolden JE, Christiansen AJ, Dickins RA, Miccolo C, Chiocca S, Kral AM, Ozerova ND, Miller TA, Methot JL, Richon VM, Secrist JP, Minucci S, Johnstone RW. Mol Cancer Ther. 2013;12:2709. doi: 10.1158/1535-7163.MCT-13-0626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.