Abstract
Introduction
Visceral adipose tissue (VAT) is a risk factor for diabetes and we investigated the amount of VAT in patients with chronic pancreatitis (CP).
Methods
Serial patients with CP seen between January 2015 and June 2016 were included in this cross-sectional, observational study. The study population was divided into alcoholic CP (group 1; N = 67) and tropical CP (group 2; N = 35). VAT was estimated using bioelectric impedance analysis (BIA) and dual energy X-ray absorptiometry (DEXA) methods. The results were analyzed by appropriate statistical methods.
Results
The study participants (85 male, 17 female) had a mean (SD) age of 40.8 (12.6) years, CP duration of 3.7 (4.7) years, and body mass index of 22.5 (3.2) kg/m2. Pancreatogenic diabetes was seen in 54 patients and the total body fat percentage was lower in the alcoholic CP group. VAT mass was similar in both the groups (p = 0.8749). CP patients with diabetes had a higher VAT mass (436 vs. 341 g) than those without diabetes (p = 0.0132). DEXA and BIA correlated in estimation of total body fat (p < 0.0001) but not in VAT (p = 0.0922).
Conclusion
VAT is a determinant in the development of diabetes, even in patients with CP. DEXA is a better modality for VAT estimation in comparison to BIA.
Keywords: BIA, Chronic pancreatitis, DEXA, Diabetes, Visceral adipose tissue
Introduction
Fat is one of the essential macronutrients for the body and is distributed mainly in subcutaneous and visceral areas [1]. This demarcation has physiological relevance because excess visceral fat has been associated with many adverse metabolic consequences [2]. Visceral fat is the main determinant of insulin resistance, and many authors have established links between excessive visceral fat and an exaggerated inflammatory state [3, 4]. The Indian phenotype is characterized by excess fat for a given body weight, which has been termed as the Y–Y paradox [5]. The accurate estimation of visceral fat is essential but difficult in clinical practice. The gold standard for the estimation of body fat distribution is the hydrostatic weighing method or air displacement plethysmography [6]; however, these techniques are not widely available and feasible to use in clinical practice. Noninvasive methods of body fat estimation include the bioelectric impedance analysis (BIA) method and the use of dual energy X-ray absorptiometry (DEXA) [7]. Out of these two methods, DEXA is considered as the gold standard for the estimation of body components in a variety of clinical conditions [8].
Chronic pancreatitis (CP) is a disorder characterized by the atrophy and calcification of the pancreas with loss of exocrine and endocrine function in varying proportions [9]. The presence of CP poses a risk of malabsorption syndrome as the pancreatic secretions are essential for the absorption of nutrients and vitamins. Diabetes secondary to pancreatic disorders has been defined as pancreatogenic diabetes, and multiple factors contribute to the risk of diabetes in patients with CP [10]. They include the amount of beta cell loss, amyloid deposits, atrophy of the pancreas, genetic risk, and advanced age. The estimation of visceral fat in patients with CP could help in predicting the natural course and the likelihood of additional factors that influence the development of diabetes. The published literature about the visceral fat distribution in patients with CP is scarce and is limited by the technique of fat estimation [11, 12]. Hence we conducted this study to evaluate the visceral fat in a large group of patients with CP with two different noninvasive methods and made a comparison according to the etiology of the CP.
Methods
Study Population
This cross-sectional, observational study was conducted at a tertiary-level armed forces hospital between January 2015 and December 2016. All patients with a known diagnosis of CP (aged 18–70) of any duration undergoing follow-up at our hospital were included in the study. We excluded patients with known systemic disorders (chronic liver disease, chronic kidney disease, thyroid disease) and long-term intake of drugs that could affect the body fat (glucocorticoids and thyroxine). We excluded patients with CP who had steatorrhea and signs of malabsorption. The patients were divided into two groups based on the etiology for the comparison: group 1 (alcoholic CP) and group 2 (tropical CP). The local ethics committee approved the trial protocol and the study, being cross-sectional and observational in nature, precluded the registration with any known database from our country. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Study Measures
A detailed history regarding the CP profile was obtained from all the participants. A general physical examination was conducted that include measurements which are relevant for the identification of abdominal obesity. Abdominal circumference (AC) was measured as the narrowest circumference between the lower costal margin and the iliac crest in centimeters. A fasting venous blood sample after an overnight fast for more than 12 h was collected from each participant at 0800 hours. The serum was analyzed for hematological and biochemical parameters. The intra- and interassay coefficient of variation for all the tests is less than 6% in our laboratory.
Body fat percentage and visceral fat were determined in the fasting state at the same time of the day by both methods using BIA and DEXA sequentially. The subjects did not exercise or consume caffeine or alcohol prior to the measurement of body fat percentage. BIA was performed using an Omron HBF-701 instrument (Omron Corporation, Shimogo-ku, Kyoto, Japan) and DEXA was performed using a Hologic QDR 2000 (Hologic ®, Bedford, MA 01730, USA). The machine calculates the percentage fat, estimated VAT in grams, and other important body composition parameters [13].
Study Definitions
Diabetes and prediabetes were defined as per the American Diabetes Association (ADA) guidelines [14]. Pancreatogenic diabetes was classically diagnosed as per the criteria proposed by Ewald and Bretzel [15]. However, we excluded patients with exocrine deficiency and patients with onset of diabetes prior to the diagnosis of CP. Hence the spectrum of diabetes evaluated in our study is a combination of T2DM and pancreatogenic diabetes. CP was diagnosed on the basis of clinical and imaging criteria [16]. Alcoholic CP was diagnosed in a patient with alcohol consumption of more than 14 units per week for 5 years prior to the onset of CP. Tropical CP (TCP) was diagnosed in patients with no identifiable cause along with normal radiological appearance of gall bladder and normal gamma-glutamyl transpeptidase (GGT).
Statistics
Data are presented as mean ± SD, and a comparison between the groups was done using nonparametric (Mann–Whitney U test) and Fisher’s exact tests. Spearman’s correlation test was used for correlation between numerical variables and a P value of less than 0.05 was considered significant. The statistical analysis and graph generation were done using the Graph Pad Prism Software, Version 6 (Graph Pad Software, San Diego, CA, USA).
Results
The study participants consist of 85 men and 17 women with a mean age of 40.8 ± 12.6 years, mean CP duration of 3.7 ± 4.7 years, body weight of 63.3 ± 10.8 kg, and mean body mass index (BMI) of 22.5 ± 3.2 kg/m2. A total of 67 patients had alcoholic CP and the remaining 35 had TCP. Pancreatogenic diabetes mellitus was seen in 54 patients with average glycosylated hemoglobin of 7.5 ± 1.6%. The antidiabetic treatment consists of metformin alone (n = 18), combination of oral drugs (n = 14), and insulin along with oral antidiabetic drugs (n = 22). Hypertension was present in 16 patients and all the patients had adequate control. The baseline parameters and the results of the body fat estimation of both groups are given in Table 1. Briefly, alcoholic CP was seen only in men and the body fat percentage was lower in the alcoholic CP group. However, the visceral fat mass was not different between the two groups. VAT was 421.9 ± 180.6 and 342.4 ± 156.9 g in men and women, respectively (p = 0.1605). Visceral fat estimated by BIA was 6.6 ± 3.9% and 7.6 ± 3.1% in men and women, respectively (p = 0.2198). The fasting serum insulin was 5.6 ± 5.5 and 7.1 ± 6.7 pmol/L in groups 1 and 2, respectively (p = 0.2739).
Table 1.
Comparison of body composition parameters between the two groups of CP
| Feature | Units | Group 1 (alcoholic CP), n = 67 | Group 2 (TCP), n = 35 | p value |
|---|---|---|---|---|
| Demographic parameters | ||||
| Age | Years | 40.4 (11.8)a | 41.4 (14.2) | 0.7114 |
| Sex | Male/female | 67:0 | 18:17 | <0.0001 |
| Duration of chronic pancreatitis | Years | 3.2 (3.2) | 4.4 (6.7) | 0.2233 |
| Diabetes mellitus | Yes/no | 40:27 | 14:21 | 0.0643 |
| Duration of diabetes mellitus | Years | 1.6 (2.8) | 2.1 (5.9) | 0.5754 |
| Weight | kg | 64.6 (10.4) | 60.8 (11.1) | 0.0927 |
| BMI | kg/m2 | 22.4 (3.2) | 22.8 (3.3) | 0.4849 |
| Abdominal circumference | cm | 84.4 (8.8) | 85.9 (9.1) | 0.4431 |
| BIA parameters | ||||
| Body fat | % | 23.4 (6.2) | 27.2 (8.3) | 0.0010 |
| Visceral fat | % | 6.4 (3.9) | 7.5 (3.6) | 0.1629 |
| Resting metabolic rate (RMR) | U/L | 1464 (179.7) | 1414 (189) | 0.1892 |
| Physiological age | Years | 39.6 (11.9) | 44.2 (15.3) | 0.0923 |
| DEXA parameters | ||||
| Body fat | % | 23.6 (4.7) | 29.6 (9.5) | <0.0001 |
| Visceral adipose tissue | g | 406.6 (169.1) | 412.5 (198.2) | 0.8749 |
| Total fat mass | g | 15,514 (5622) | 18,764 (7153) | 0.0133 |
| Android/gynoid ratio | Number | 1.07 (0.19) | 1.02 (0.18) | 0.1609 |
Bold indicate significant p values
aMean (SD)
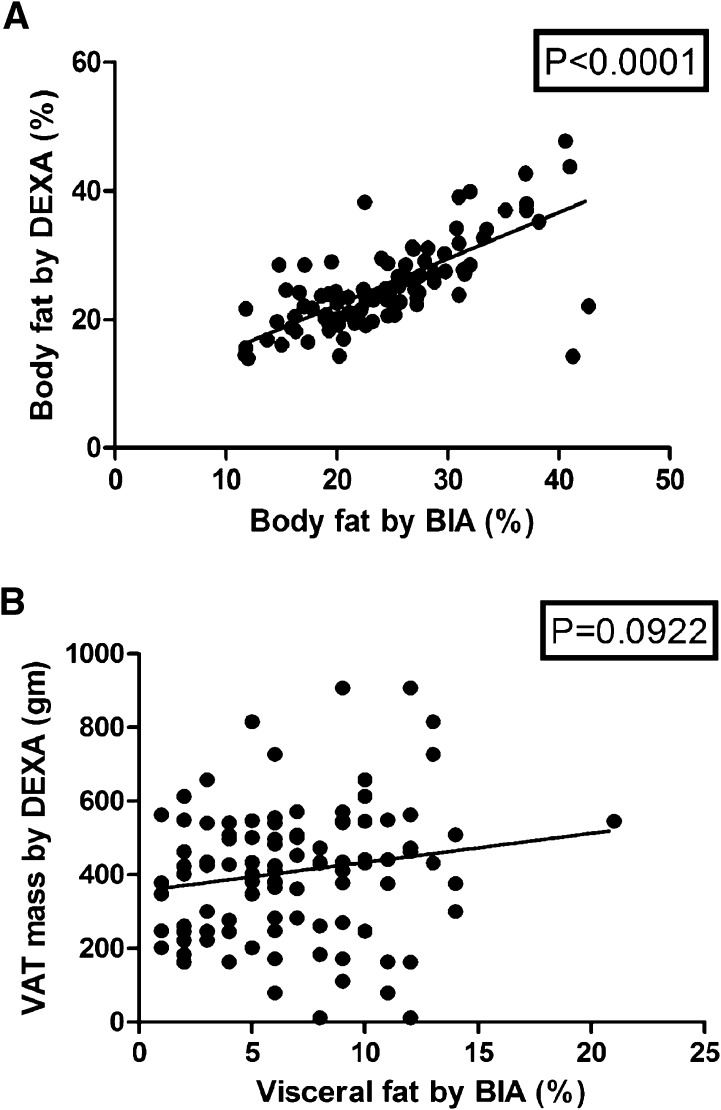
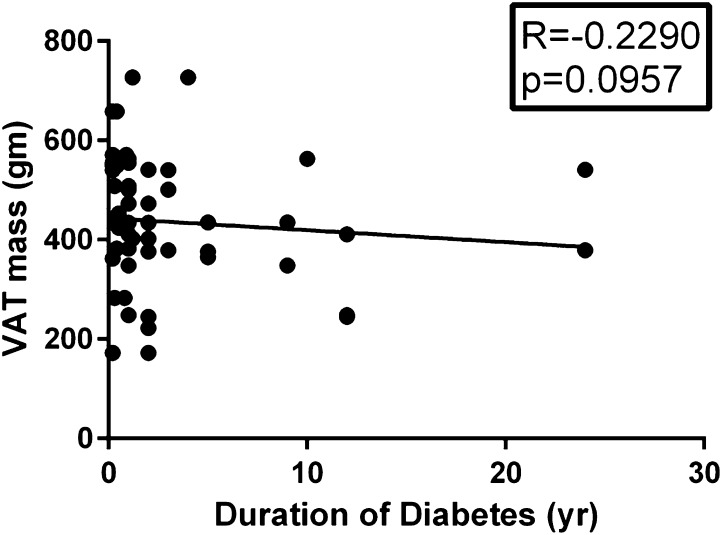
We analyzed the body fat distribution as per the status of the diabetes and the results are shown in Table 2. Briefly, the total body fat percentage was the same, but the percentage of visceral fat was higher in the patients with diabetes. The patients with diabetes had a lower resting metabolic rate (RMR) in comparison to patients without diabetes. BIA and DEXA showed a linear correlation with regard to the body fat estimation, but did not match in the visceral fat estimation as shown in Fig. 1. The RMR showed a negative correlation with advancing age (p = 0.0026). Visceral adipose tissue by DEXA did not show any correlation with age (p = 0.5090). The adipose tissue parameters did not show any correlation with the duration of diabetes as shown in Fig. 2.
Table 2.
Comparison of parameters between patients with and without diabetes
| Feature | Units | Group 1 (DM present), n = 54 | Group 2 (DM absent), n = 48 | p value |
|---|---|---|---|---|
| BIA parameters | ||||
| Body fat | % | 24 (7)a | 25.4 (7.4) | 0.3342 |
| Visceral fat | % | 6 (3.2) | 7.6 (4.2) | 0.0349 |
| Resting metabolic rate (RMR) | U/L | 1410 (181.7) | 1488 (186.7) | 0.0327 |
| Physiological age | Years | 40.7 (10.9) | 41.7 (15.6) | 0.6887 |
| DEXA parameters | ||||
| Body fat | % | 24.9 (6.8) | 26.5 (7.7) | 0.2842 |
| Visceral adipose tissue | g | 435.5 (132.2) | 341.4 (181.3) | 0.0032 |
| Total fat mass | g | 16,250 (6008) | 17,057 (6745) | 0.5244 |
| Android/gynoid ratio | Number | 1.04 (0.13) | 1.06 (0.22) | 0.5558 |
Bold indicate significant p values
aMean (SD)
Fig. 1.
Correlation between BIA and DEXA regarding fat percentage
Fig. 2.
Correlation between VAT and the duration of diabetes
Discussion
Our study showed a few interesting findings in the body composition and VAT mass of CP patients. The total body fat was lower in patients with alcoholic CP in comparison to those with TCP. This is self-explanatory as the patients consuming alcohol are prone to malabsorption and have less body fat [17]. Patients with long-standing alcohol intake are more prone to malnutrition and have an increased risk of vitamin and mineral deficiency [18]. The malnutrition in CP could be due to multiple factors like poor oral intake, duodenal obstruction, secondary diabetes, and increased metabolic activity [19]. However, the interesting finding is that the patients with alcoholic CP have the same amount of VAT in comparison to those with TCP. This suggests that VAT is genetically determined and not influenced by external factors. Previous research has shown that the visceral fat is determined by the waist circumference and certain transcription factors [20, 21].
Our data showed that the patients with DM had a low RMR when compared with the controls. RMR is the rate of the resting energy expenditure that is required by the body without any activity. High RMR is a characteristic finding of the active inflammatory state in acute pancreatitis [22]. We excluded patients with exocrine dysfunction and acute pancreatitis, thereby limiting the role of RMR assessment. The low RMR could have contributed to the high prevalence of VAT in patients with diabetes in our study. We used BIA for the estimation of RMR, which has been shown to be comparable with RMR derived from the Harris–Benedict equation [23]. The average predicted RMR was 1371 kcal/day in that study, which is comparable to our data. Another study showed that patients with a BMI less than 20 kg/m2 had a higher predicted RMR in alcoholic CP [24]. Malnutrition is a common component in the patients with CP and calculation of the RMR is essential in planning the nutritional requirement of the patients.
Another interesting finding from our study is the observed discrepancy between BIA and DEXA in the estimation of the total body fat and VAT (Fig. 1). This could be explained by the lack of sensitivity of the BIA method for the estimation of VAT. BIA is a method that is based on the passage of an electric current in the body tissue and is affected by a wide variety of factors [25]. DEXA is a better modality and is often comparable to hydrodensitometry or air displacement plethysmography [26]. Hence, it is recommended that DEXA should be used for the estimation of VAT, rather than the BIA method. Few studies have looked at the correlation of VAT and subcutaneous adipose tissue (SAT) with pancreatic exocrine function [27]. Body composition studies have shown that pancreatic exocrine function is closely linked to muscle mass and sarcopenia [28]. However, we excluded all patients with exocrine dysfunction to study the role of VAT in endocrine dysfunction alone.
The strengths of our study include assessment of VAT by two methods commonly available in clinical practice and identifying the relevance of VAT estimation in the management of CP. The limitations of our study include the small sample size, lack of comparison of body composition parameters with the gold standard method (hydrostatic weighing or air displacement plethysmography), and the data being derived from a single center and thereby may not be applicable to the entire population. The cross-sectional nature of our study limits the usefulness in predicting the cause and effect relation between VAT and diabetes in CP. Another limitation was the exclusion of patients with exocrine deficiency and thereby being unable to decipher the effect of insulin on exocrine functions of the pancreas.
Conclusion
VAT is one of the determinants in the development of diabetes, even in patients with CP. DEXA is a better modality for VAT estimation in comparison to BIA. Further large-scale studies with larger numbers of patients are required to confirm the findings observed in our study.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. KVS Hari Kumar designed the study. Rahul Sharma and Manish Manrai analyzed the data and did statistical analyses. AK Sood created the figures and KVS Hari Kumar wrote the report. All the authors read and revised the report, and approved the final submitted version. KVS Hari Kumar assumes responsibility for the completeness and accuracy of the data and analyses.
Disclosures
KVS Hari Kumar, Rahul Sharma, Manish Manrai, and AK Sood have nothing to disclose.
Compliance with Ethics Guidelines
This article is compliant with all the ethical guidelines and permission was obtained from the institutional ethical committee for the study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available because of the data pertaining to the military service personnel but are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/590CF06028140311.
References
- 1.Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55(10):2622–2630. doi: 10.1007/s00125-012-2639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One. 2013;8(2):e56415. doi: 10.1371/journal.pone.0056415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer K, Pick JA, Moewes D, Nöthlings U. Qualitative aspects of diet affecting visceral and subcutaneous abdominal adipose tissue: a systematic review of observational and controlled intervention studies. Nutr Rev. 2015;73(4):191–215. doi: 10.1093/nutrit/nuu006. [DOI] [PubMed] [Google Scholar]
- 4.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50(4):425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 5.Yajnik CS, Yudkin JS. The Y-Y paradox. Lancet. 2004;363(9403):163. doi: 10.1016/S0140-6736(03)15269-5. [DOI] [PubMed] [Google Scholar]
- 6.Heyward VH. Evaluation of body composition. Current issues. Sports Med. 1996;22(3):146–156. doi: 10.2165/00007256-199622030-00002. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher D, Shaheen I, Zafar K. State-of-the-art measurements in human body composition: a moving frontier of clinical importance. Int J Body Compos Res. 2008;6(4):141–148. [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson Stoklossa CA, Forhan M, Padwal RS, Gonzalez MC, Prado CM. Practical considerations for body composition assessment of adults with class II/III obesity using bioelectrical impedance analysis or dual-energy X-ray absorptiometry. Curr Obes Rep. 2016;5(4):389–396. doi: 10.1007/s13679-016-0228-5. [DOI] [PubMed] [Google Scholar]
- 9.Majumder S, Chari ST. Chronic pancreatitis. Lancet. 2016;387(10031):1957–1966. doi: 10.1016/S0140-6736(16)00097-0. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta R, Naik D, Thomas N. Emerging concepts in the pathogenesis of diabetes in fibrocalculous pancreatic diabetes. J Diabetes. 2015;7(6):754–761. doi: 10.1111/1753-0407.12280. [DOI] [PubMed] [Google Scholar]
- 11.Singla MK, Mukhopadhyay P, Pandit K, Chowdhury S. A clinical profile of fibrocalculous pancreatic diabetes patients from eastern India with special reference to body fat percentage and insulin resistance. J Indian Med Assoc. 2009;107(11):762–764. [PubMed] [Google Scholar]
- 12.Hébuterne X, Hastier P, Péroux JL, Zeboudj N, Delmont JP, Rampal P. Resting energy expenditure in patients with alcoholic chronic pancreatitis. Dig Dis Sci. 1996;41(3):533–539. doi: 10.1007/BF02282334. [DOI] [PubMed] [Google Scholar]
- 13.Snijder MB, Visser M, Dekker JM, et al. The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: a comparison with computed tomography and anthropometry. Int J Obes Relat Metab Disord. 2002;26(7):984–993. doi: 10.1038/sj.ijo.0801968. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care. 2014;37:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 15.Ewald N, Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (type 3c)—are we neglecting an important disease? Eur J Intern Med. 2013;24(3):203–206. doi: 10.1016/j.ejim.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Shimosegawa T, Kataoka K, Kamisawa T, et al. The revised Japanese clinical diagnostic criteria for chronic pancreatitis. J Gastroenterol. 2010;45(6):584–591. doi: 10.1007/s00535-010-0242-4. [DOI] [PubMed] [Google Scholar]
- 17.Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Influence of chronic alcohol abuse on body weight and energy metabolism: is excess ethanol consumption a risk factor for obesity or malnutrition? J Intern Med. 1998;244(5):387–395. doi: 10.1046/j.1365-2796.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 18.Ross LJ, Wilson M, Banks M, Rezannah F, Daglish M. Prevalence of malnutrition and nutritional risk factors in patients undergoing alcohol and drug treatment. Nutrition. 2012;28(7–8):738–743. doi: 10.1016/j.nut.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Afghani E, Sinha A, Singh VK. An overview of the diagnosis and management of nutrition in chronic pancreatitis. Nutr Clin Pract. 2014;29(3):295–311. doi: 10.1177/0884533614529996. [DOI] [PubMed] [Google Scholar]
- 20.Giusti V, Suter M, Verdumo C, Gaillard RC, Burckhardt P, Pralong FP. Molecular determinants of human adipose tissue: differences between visceral and subcutaneous compartments in obese women. J Clin Endocrinol Metab. 2004;89(3):1379–1384. doi: 10.1210/jc.2003-031507. [DOI] [PubMed] [Google Scholar]
- 21.Glueck CJ, Wang P, Woo JG, Morrison JA, Khoury PR, Daniels SR. Adolescent and young adult female determinants of visceral adipose tissue at ages 26–28 years. J Pediatr. 2015;166(4):936-46.e1-3. doi: 10.1016/j.jpeds.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 22.Habtezion A. Inflammation in acute and chronic pancreatitis. Curr Opin Gastroenterol. 2015;31(5):395–399. doi: 10.1097/MOG.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skallerup A, Nygaard L, Olesen SS, Vinter-Jensen L, Køhler M, Rasmussen HH. Can we rely on predicted basal metabolic rate in patients with intestinal failure on home parenteral nutrition? JPEN J Parenter Enteral Nutr. 2016. doi:10.1177/0148607116657648. [DOI] [PubMed]
- 24.Hebuterne X, Hastier P, Peroux JL, Zeboudj N, Delmont JP, Rampal P. Resting energy expenditure in patients with alcoholic chronic pancreatitis. Dig Dis Sci. 1996;41:533–539. doi: 10.1007/BF02282334. [DOI] [PubMed] [Google Scholar]
- 25.Vicente-Rodríguez G, Rey-López JP, Mesana MI, et al. Reliability and intermethod agreement for body fat assessment among two field and two laboratory methods in adolescents. Obesity (Silver Spring) 2012;20(1):221–228. doi: 10.1038/oby.2011.272. [DOI] [PubMed] [Google Scholar]
- 26.Wagner DR, Heyward VH. Techniques of body composition assessment: a review of laboratory and field methods. Res Q Exerc Sport. 1999;70(2):135–149. doi: 10.1080/02701367.1999.10608031. [DOI] [PubMed] [Google Scholar]
- 27.Haaber AB, Rosenfalck AM, Hansen B, Hilsted J, Larsen S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int J Pancreatol. 2000;27:21–27. doi: 10.1385/IJGC:27:1:21. [DOI] [PubMed] [Google Scholar]
- 28.Shintakuya R, Uemura K, Murakami Y, et al. Sarcopenia is closely associated with pancreatic exocrine insufficiency in patients with pancreatic disease. Pancreatology. 2017;17(1):70–5. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available because of the data pertaining to the military service personnel but are available from the corresponding author on reasonable request.