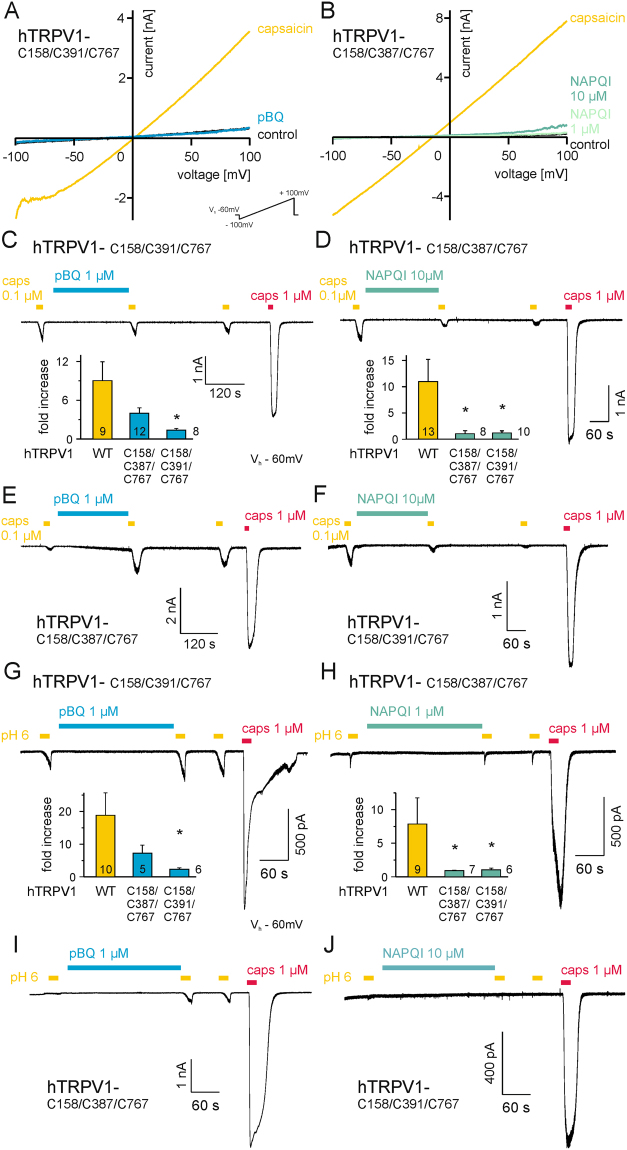
Figure 6.
Modulation of cysteines underlies the sensitization of TRPV1 by pBQ and NAPQI. (A,B) Mutation of internal cysteines in hTRPV1 abolishes sensitization of by both pBQ (G C158A/C391S/C767S-hTRPV1) and NAPQI (H C158A/C387S/C767S-hTRPV1). pBQ and both concentrations of NAPQI were applied for five minutes. These cysteines are also involved in sensitization of capsaicin- (C–F) and proton-induced (G–J) inward currents by both metabolites. Representative traces are displayed for pBQ (C and G, C158S/C391S/C767S-hTRPV1; (E and I) C158A/C387S/C767S-hTRPV1) and NAPQI (D and H), C158A/C387S/C767S-hTRPV1; F and J, C158S/C391S/C767S-hTRPV1). Bar diagrams show multiples of capsaicin- or proton-induced responses before and after application of pBQ (C and G) or NAPQI (D and H) in wild-type and mutant hTRPV1. Sensitization of capsaicin- and proton-induced responses was reduced for pBQ in C158A/C391S/C767S-hTRPV1 (*p ≤ 0.007), and completely lost for NAPQI in both mutants (*p ≤ 0.0009 respectively *p ≤ 0.005).