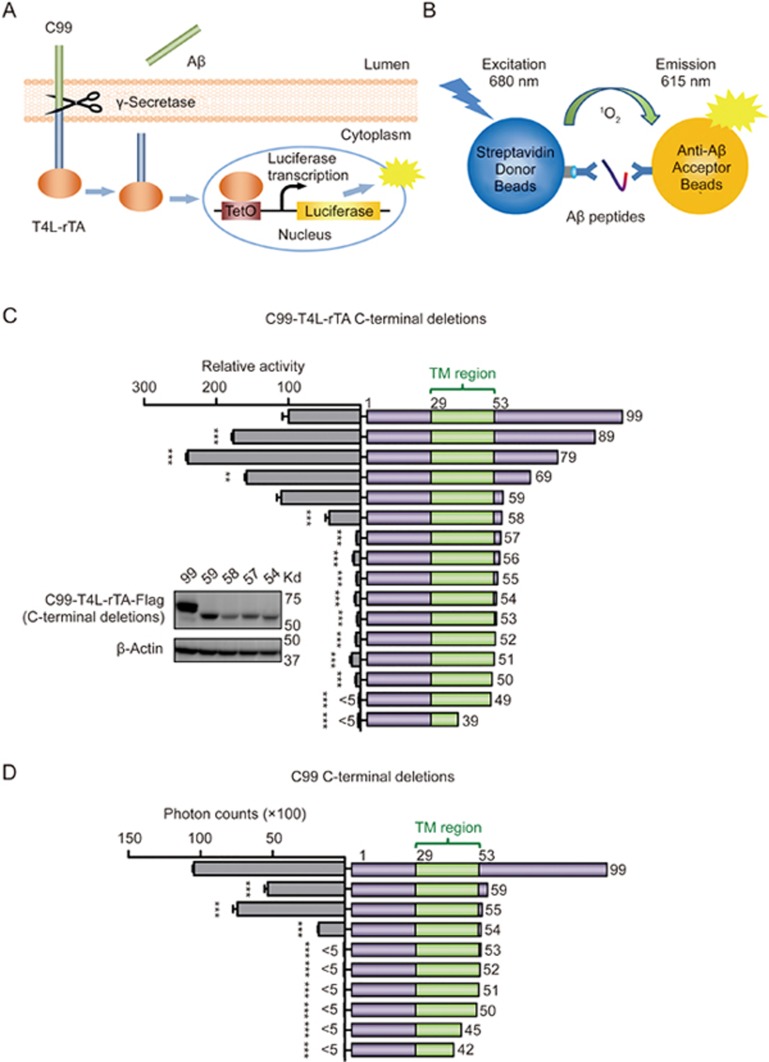
Figure 1.
Defining the C99 minimum boundary for γ-secretase cleavage. (A) An overall view of the γ-secretase Epsilon-Cleavage assay. Once C99-T4L-rTA hybrid protein is cleaved by endogenous γ-secretase, Aβ peptides are released into the culture medium, and the AICD-T4L-rTA hybrid protein is translocated into the nucleus, where it binds tetO and activates luc transcription to generate a luciferase signal as measurement of total C99 cleavage. (B) An overall view of the AlphaLISA assay. The biotinylated anti-Aβ antibody binds to Streptavidin-coated donor beads, and AlphaLISA acceptor beads are directly conjugated to the anti-Aβ antibody. The presence of Aβ peptides brings the two beads into close proximity to generate a light emission signal as measurement of secreted Aβ levels. (C and D) Defining the C-termini of the minimum substrate by γ-secretase Epsilon-Cleavage assay (C) and AlphaLISA assay (D). Cartoon illustration of the C-terminally truncated C99 fragments (right side) and their cleavage efficiencies (left side). The numbers in the cartoon illustration indicate the last residue in each construct. The inlet panel of C shows relative protein expression of some critical boundary constructs, with corresponding construct numbers marked on top of each lane. Aβ55, which contains first 55 amino acids of C99, is the shortest substrate among these C99 C-terminal truncations that retained similar activity as wild-type. Error bars=SEM, n=3, P-values (two-tailed Student's t-test versus WT): *P<0.05; **P<0.01, ***P<0.001.