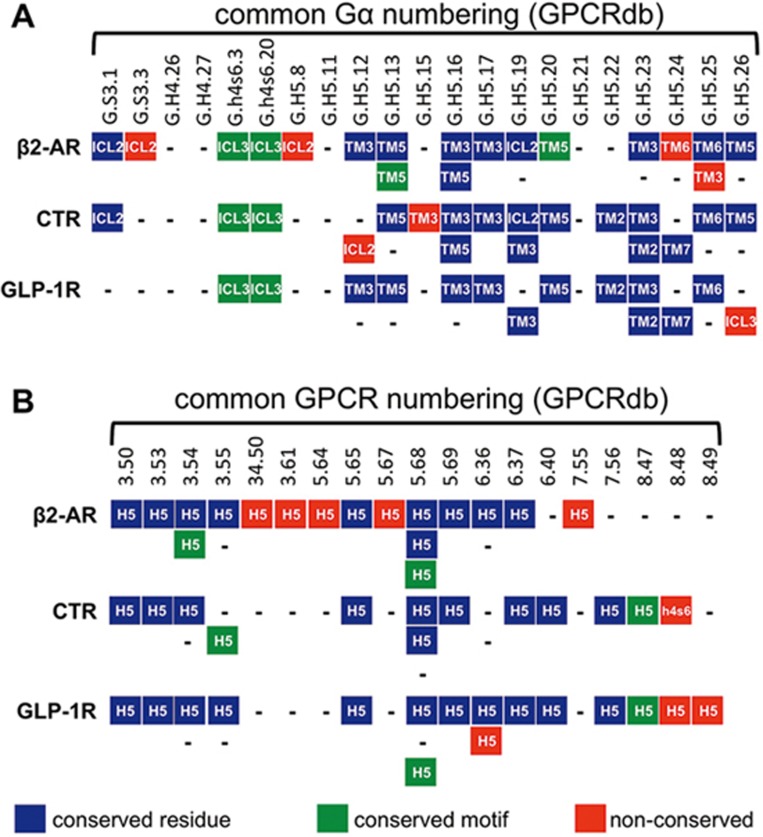
Figure 1.
Selectivity determinants from GPCRdb were mapped onto Gαsβγ:β2AR (3sn6), Gαsβγ:CTR (5uz7) and Gαsβγ:GLP-1R (5vai), and residues within ∼4 Å and showing an appropriate interaction were manually annotated (other residues are not shown). In A, residues from the Gα interface are mapped against the 3 receptors. Gα positions are labeled using the common Gα numbering system (GPCRdb) where S3 is β-strand 3, h4s6 is the loop between helix 4 and β-strand 6 and H5 is helix 5 (all in the Ras-like domain). These are shown against interacting residues in the 3 GPCRs; in blue are GPCR residues that have corresponding positions (according to GPCRdb), green are GPCR residues in corresponding secondary structures but at alternative positions and red illustrates unique GPCR residues. In B, residues from the G protein-coupling pocket are mapped against GPCRs (only interacting residues are shown); the color scheme is the same as A but applies to Gαs. Pocket residues are numbered according to GPCRdb in which the first (single) number indicates the transmembrane helix or, in the case of 2 numbers, the loop connecting 2 helices. The number to the right of the decimal indicates the position of this residue relative to the most conserved helix residue, which is given the value of 50.