Abstract
Objective:
To examine the association between sepsis and the long-term risk of seizures.
Methods:
We conducted a retrospective population-based cohort study using administrative claims data from all emergency department visits and hospitalizations at nonfederal acute care hospitals in California, Florida, and New York from 2005 to 2013. Using previously validated diagnosis codes, we identified all adult patients hospitalized with sepsis. Our outcome was any emergency department visit or hospitalization for seizure. Poisson regression and demographic data were used to calculate age-, sex-, and race-standardized incidence rate ratios (IRR). To confirm our findings, we used a matched cohort of hospitalized patients without sepsis for comparison and additionally assessed claims data from a nationally representative 5% sample of Medicare beneficiaries.
Results:
We identified 842,735 patients with sepsis. The annual incidence of seizure was 1.29% (95% confidence interval [CI] 1.27%–1.30%) in patients with sepsis vs 0.16% (95% CI 0.16%–0.16%) in the general population (IRR 4.98; 95% CI 4.92–5.04). A secondary analysis using matched hospitalized patients confirmed these findings (IRR 4.33; 95% CI 4.13–4.55), as did a separate analysis of Medicare beneficiaries, in whom we found a similar strength of association (IRR 2.72; 95% CI 2.60–2.83), as we did in patients ≥65 years of age in our primary statewide data (IRR 2.83; 95% CI 2.78–2.88).
Conclusions:
We found that survivors of sepsis faced a significantly higher long-term risk of seizures than both the general population and other hospitalized patients. Our findings suggest that sepsis is associated with pathways that lead to permanent neurologic sequelae.
Sepsis is a life-threatening inflammatory response to infection that causes organ dysfunction.1 Neurologic complications during sepsis are common and include sepsis-associated encephalopathy, seizures, stroke, and neuromuscular disease.2 Sepsis-associated encephalopathy is possibly the most common and has led to the recognition of long-term cognitive dysfunction after sepsis,3–6 while increasing use of continuous EEG has led to more widespread recognition of seizures in critically ill patients with sepsis. Animal models have suggested multiple possible causes for the development of these cerebral sequelae, including disruption of the blood–brain barrier7 leading to an inflammatory response in the CNS,8,9 altered cerebral blood flow and metabolism,9 apoptosis,10 mitochondrial dysfunction,11 and inhibition of neurovascular coupling.12 In addition, patients with sepsis may be at risk of silent cerebral infarcts or kindling from nonconvulsive seizures. Despite these insights, it is not known whether survivors of sepsis continue to face an increased long-term risk of developing seizures after hospital discharge. We hypothesized that patients who survive a hospitalization for sepsis have a higher long-term risk of seizures and tested this hypothesis in a retrospective, population-based study using complementary sets of administrative claims data.
METHODS
Design.
We compared the rate of seizures in survivors of sepsis vs (1) the general population and (2) a matched cohort of patients hospitalized without sepsis. To do so, we used administrative claims data on all discharges from nonfederal emergency departments (EDs) and acute care hospitals in California from 2005 through 2011, New York from 2006 through 2013, and Florida from 2005 through 2013. These statewide data were collected by the California Office of Statewide Health Planning and Development, the New York Statewide Planning and Research Cooperative System, and the Florida Agency for Health Care Administration. These 3 agencies provide these data to the Agency for Healthcare Research and Quality for its Healthcare Cost and Utilization Project.13 Each patient is assigned a personal linkage number that allows him or her to be followed anonymously through all subsequent hospitalizations.14 Up to 25 discharge diagnoses, labeled as present before admission or developed during hospitalization, are coded at each encounter using the ICD-9-CM system. In addition, we performed a confirmatory analysis of the same exposure and outcome in Medicare beneficiaries. The US federal government's Centers for Medicare and Medicaid Services (CMS) provides health insurance to a large majority of US residents once they reach 65 years of age. CMS makes available to researchers datasets that lack identifiable patient information but include data on claims submitted by providers and hospitals in the course of Medicare beneficiaries' clinical care.15 Each claim includes the dates of service and up to 25 ICD-9-CM diagnosis codes justifying the claim. Multiple claims for a given patient can be linked via a unique, anonymous identifier code, thus allowing for a comprehensive and longitudinal analysis of each beneficiary’s care over time.
Standard protocol approvals, registrations, and patient consents.
The institutional review board at Weill Cornell Medical College approved this study and waived the requirement for informed consent.
Participants.
Participants in the statewide analysis.
Using standard ICD-9-CM codes in an algorithm defined by Dombrovskiy et al.,16,17 we used codes for sepsis plus those for organ dysfunction (table e-1 at Neurology.org) to identify all patients over the age of 18 who were hospitalized with sepsis in California, Florida, and New York. The Dombrovskiy et al. algorithm was initially designed for the identification of severe sepsis in population-based studies, but subsequent studies have shown that this algorithm most closely corresponds to the overall burden of sepsis in the United States as ascertained by chart-based studies.18 Since we were interested in the long-term risk of new-onset seizures after sepsis, diagnoses of seizure prior to or during the index sepsis hospitalization were excluded. To maximize longitudinal follow-up data, we excluded nonresidents of the 3 states listed. Finally, we excluded patients who died during the index hospitalization for sepsis.
Following the approach of prior studies on the long-term risk of epilepsy,19 we compared the risk of seizures in those with sepsis vs the general population. Our estimates of the rate of seizures in the general population were based on discharge diagnoses of seizures from EDs and hospitals, divided by the time at risk and the total population as determined from publicly available demographic data from the 3 states included in this analysis.20 This approach was made possible because these statewide data sources captured essentially all discharges in their respective states and because almost all patients with first-time seizures present for evaluation in a hospital or ED setting.19,21 In addition to comparing sepsis survivors to the general population, we performed an analysis comparing sepsis survivors to survivors of hospitalizations for another diagnosis besides sepsis. These patients were matched based on age, sex, race, insurance, length of stay, discharge location, year of hospitalization, state, and the presence of corresponding codes for organ dysfunction per the Dombrovskiy et al. algorithm (neurologic, respiratory, cardiovascular, renal, hepatic, hematologic, or metabolic).22 Using New York data, which include a reliable indicator of admission to an intensive care unit (ICU), we performed an additional analysis also matched by (1) ICU status, (2) whether patients received mechanical ventilation, a useful surrogate of illness severity, and (3) Elixhauser comorbidities.
Participants in the Medicare analysis.
We also assessed the relationship between sepsis and long-term seizure risk using inpatient and outpatient claims data from 2008 through 2014 from a nationally representative 5% sample of Medicare beneficiaries. In keeping with standard practice in analyzing Medicare data,23 we limited our cohort to beneficiaries with continuous coverage in traditional fee-for-service Medicare (both parts A and B) for at least 1 year. Since Medicare eligibility generally begins at 65 years of age, we included only patients ≥66 years of age in order to allow time for beneficiaries to enter medical care and for their providers to document any preexisting comorbidities. Sepsis was identified using the same algorithm as in our statewide analysis, and we again excluded diagnoses of seizure prior to or during the index sepsis hospitalization, as well as patients who died during the index hospitalization for sepsis. The risk of seizures among sepsis survivors was compared to Medicare beneficiaries without sepsis.
Measurements.
Outcomes.
In the statewide data, our primary outcome was any ED visit or hospitalization for seizure, which we defined as any ICD-9-CM code for epilepsy (345.x), an approach that has previously been shown to have a positive predictive value ranging from 84% to 98% in adult patients.24–26 In a sensitivity analysis, we also included in our outcome variable a less well-validated but commonly used ICD-9-CM code for convulsions (780.39). A secondary outcome was status epilepticus that resulted in hospitalization and was present at the time of admission. In the Medicare analysis, our outcome of seizure was defined as ≥1 inpatient claim with an ICD-9-CM code for epilepsy (345.x) or ≥2 such outpatient claims within 3 months of each other.
Covariates.
When comparing the risk of seizures in those with sepsis vs the general population of California, Florida, and New York, we adjusted for age, sex, and race. In the matched analysis of those with hospitalization for sepsis vs hospitalization for another diagnosis, we additionally adjusted for state and year of hospitalization, insurance status, organ dysfunction (table e-1), length of stay, and discharge disposition. In a matched analysis of patients in New York, we additionally matched for ICU admission, mechanical ventilation (ICD-9-CM codes 96.7x27), and Elixhauser comorbidities.22
In the primary Medicare analysis, we adjusted for age, sex, and race. In a sensitivity analysis, we also used ICD-9-CM codes to identify and exclude patients with the following well-established risk factors for seizure: stroke, traumatic brain injury, CNS infection (meningitis, encephalitis, and brain abscess), and brain neoplasm.
Statistical analysis.
We used standard descriptive statistics with exact confidence intervals (CIs) to report crude rates. Baseline characteristics were compared using the χ2 test and t test. Survival statistics were used to calculate incidence rates of seizure per 100,000 patients per year. Poisson regression was used to calculate incidence rate ratios (IRRs). Based on the results of our confirmatory analysis in Medicare beneficiaries, we performed a post hoc subgroup analysis of our primary data stratified by age <65 years vs ≥65 years. All statistical analyses were performed by H.K. using SAS version 9.3 (Cary, NC) and STATA/MP version 14 (College Station, TX). The threshold of statistical significance for all analyses was set at α = 0.05.
RESULTS
Statewide analysis.
Comparison with the general population.
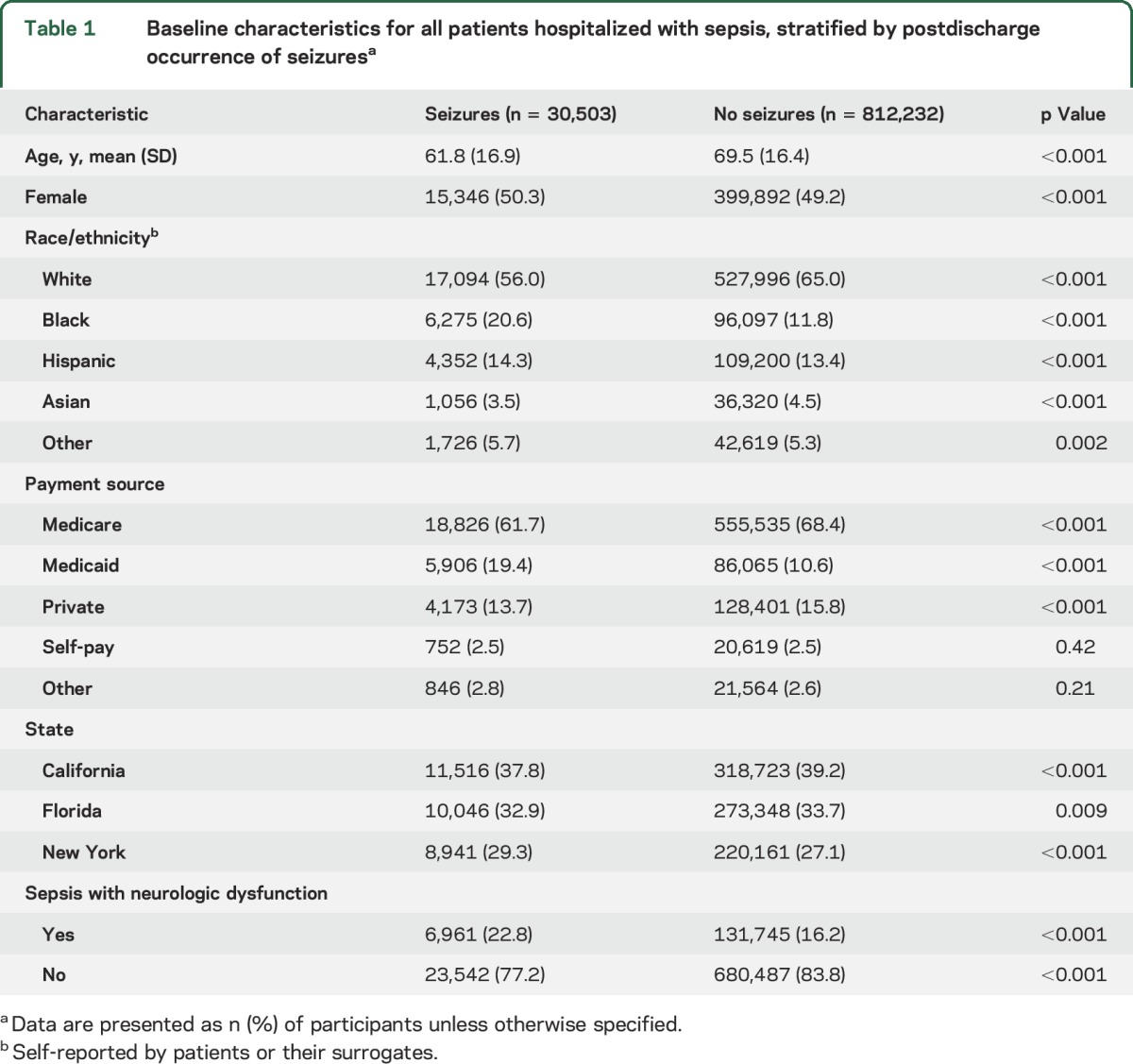
We identified 842,735 patients hospitalized with sepsis in California, Florida, and New York between 2005 and 2013. Their mean age was 69.2 (±16.5) years, 50.7% were male, and 64.7% were white. The crude rate of any seizure after hospital discharge was 3.62% (95% CI 3.58%–3.66%) (table e-2). Among patients with sepsis, those with subsequent seizures were significantly younger and more likely to have had concurrent neurologic dysfunction during the index sepsis hospitalization than those who did not have subsequent seizures (table 1).
Table 1.
Baseline characteristics for all patients hospitalized with sepsis, stratified by postdischarge occurrence of seizuresa
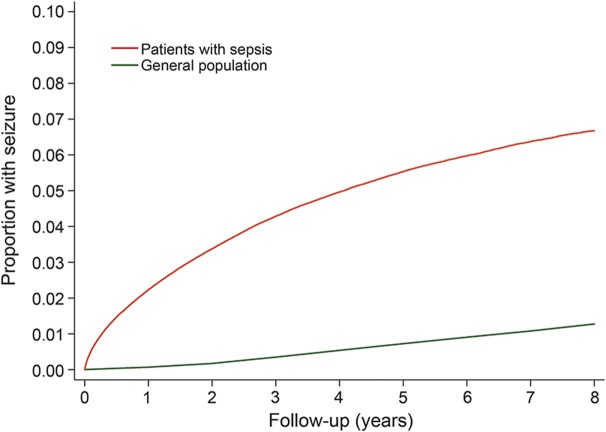
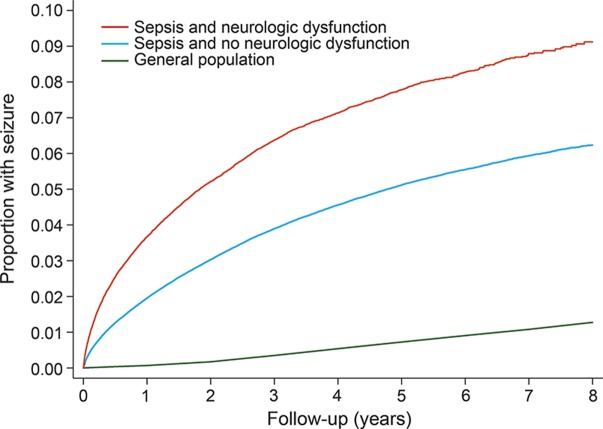
The annual incidence of seizures was 1.29% (95% CI 1.27%–1.30%) in patients with sepsis, as compared with 0.16% (95% CI 0.16%–0.16%) among the general population of California, Florida, and New York (IRR 4.98; 95% CI 4.92–5.04). By 8 years, the cumulative rate of any ED visit or hospitalization for seizure was 6.67% (95% CI 6.57%–6.78%) after discharge with sepsis, as compared with 1.27% (95% CI 1.27%–1.28%) in the general population (figure 1). The association between sepsis and subsequent seizure was strongest in those with concurrent neurologic dysfunction during hospitalization with sepsis (IRR 7.52; 95% CI 7.35–7.71), but it was also significant in those without documented neurologic dysfunction (IRR 4.53; 95% CI 4.47–4.59) (figure 2). Sepsis was associated with seizures in a sensitivity analysis that also included ICD-9-CM code 780.39 in the outcome variable (IRR 3.93; 95% CI 3.88–3.98). Sepsis was also associated with the secondary outcome of prehospital status epilepticus resulting in hospitalization (IRR 5.42; 95% CI 4.91–5.98).
Figure 1. Cumulative rates of seizures among survivors of sepsis hospitalization vs the general population.
Figure 2. Cumulative rates of seizures among survivors of sepsis hospitalization, with and without concurrent neurologic dysfunction, vs the general population.
Comparison with a matched hospitalized cohort.
In a cohort of hospitalized controls matched to sepsis survivors based on demographics, discharge disposition, length of stay, and the degree of organ dysfunction, the annual incidence of seizures was 0.25% (95% CI 0.25%–0.27%) among those without sepsis compared to 1.10% (95% CI 1.07%–1.14%) in those with sepsis. As compared to matched hospitalized patients rather than to the general population, patients with sepsis were still more likely to develop subsequent seizures (IRR 4.33; 95% CI 4.13–4.55). Our findings were similar after further matching for ICU admission, mechanical ventilation, and Elixhauser comorbidities (IRR 4.64; 95% CI 3.68–5.84).
Medicare analysis.
In a confirmatory analysis using separate data on Medicare beneficiaries, hospitalization for sepsis was again associated with subsequent seizures (IRR 2.72; 95% CI 2.60–2.83). This result was largely unchanged in a sensitivity analysis that excluded patients with stroke, traumatic brain injury, CNS infection, and brain neoplasm (IRR 2.18; 95% CI 2.06–2.32).
Post hoc analysis.
Given the difference in the strength of association between sepsis and seizures in our statewide analysis vs the Medicare analysis, we performed a post hoc subgroup analysis of the statewide data stratified by age. In patients ≥65 years of age, the association between sepsis and postdischarge seizures was similar to what we found in the Medicare population (IRR 2.83; 95% CI 2.78–2.88). Among patients <65 years of age, sepsis was much more strongly associated with subsequent seizures (IRR 10.33; 95% CI 10.17–10.49). A test of interaction confirmed that the association between sepsis and seizures varied significantly by age (p < 0.001).
DISCUSSION
We found that survivors of sepsis faced a significant long-term risk of developing seizures, with a risk that was approximately 5 times higher than the general population. Patients with sepsis had an increased long-term risk of seizures even compared to other hospitalized patients. The association between sepsis and seizures was strongest in patients with concurrent neurologic dysfunction, but even those without any documented neurologic deficits at the time of sepsis hospitalization were at an increased risk, raising the possibility that sepsis itself may represent a previously unrecognized seizure risk factor.
Although prior studies have reported an increased risk of developing long-term cognitive dysfunction after sepsis,5,28 it remains unclear how much of this was due to preexisting cognitive impairment, frailty, and the lingering effects of sedative medications, as opposed to permanent structural brain injury.29 The association we found between sepsis and subsequent seizures implicates sepsis in pathways of brain injury that in turn lead to long-term sequelae. Previous retrospective studies have found that 11%–16% of patients with severe sepsis have electrographic seizures on continuous EEG during their hospitalization,30,31 but they did not follow these patients beyond their hospitalization. Our study builds on these findings, demonstrating that patients with sepsis are at risk of developing seizures even after hospital discharge. In addition to shedding light on the natural history of sepsis, this represents a novel finding from a neurologic perspective, as a history of sepsis is not a currently recognized risk factor for seizures.32
Our study has several limitations. First, we relied on ICD-9-CM codes to identify patients with sepsis and seizures, which may have resulted in misclassification of our exposure and outcome. We took care to use previously validated diagnosis codes for both sepsis and seizures. Furthermore, miscoding of sepsis or seizures would most likely be nondifferential and thus bias our study towards the null finding of no association between sepsis and seizures. Second, in our primary analysis, we were only able to identify seizures resulting in an ED visit or hospitalization. However, this is unlikely to have substantially affected our results. Most first-time seizures result in at least an ED visit or hospitalization19,21; in support of this, the overall incidence of seizures we found in the general population was in line with previous reports of seizure incidence.33–40 More importantly, our estimates of the association between sepsis and seizure were nearly identical when we analyzed Medicare claims that included data on outpatient diagnoses. Third, we lacked data on clinical characteristics such as sepsis severity, the characteristics of infections and treatments, and EEG and neuroimaging findings. Thus, we cannot relate the relative risk of seizures to these baseline measures. Fourth, patients with sepsis may have had other coexisting CNS pathologies that contributed to the development of seizures. However, this is unlikely to substantially explain our results because our findings were largely unchanged in a sensitivity analysis excluding patients with established seizure risk factors, namely stroke, traumatic brain injury, meningitis/encephalitis, and brain tumors. Other medical complications that are common in patients with sepsis, such as renal failure, may also have caused residual confounding. However, our findings were also essentially unchanged when patients with sepsis were compared to other similar hospitalized patients, including those who were matched based on illness severity, organ dysfunction, and comorbidities. Fifth, the association between sepsis and seizures may have been partially due to ascertainment bias, in that seizure episodes may have been more likely to be detected and documented in patients with recent sepsis—a group in close contact with medical providers—than the general population. However, we found a similar association between sepsis and out-of-hospital status epilepticus, a life-threatening form of seizures that is unlikely to suffer from ascertainment bias. Sixth, given the lack of data on medication use, we were unable to account for antiepileptic drug treatments that may have influenced the risk of developing seizures. It is possible that some patients may have been started on antiseizure prophylaxis at some point during or after their hospitalization for sepsis. However, such practice is unlikely to be common, and to the degree that it occurred, it would have led to an underestimation of seizure incidence after sepsis. On the other hand, some of the increased seizure risk after sepsis hospitalization may have been related to withdrawal of certain medicines such as benzodiazepine sedatives. However, we found an association between sepsis and seizures that persisted over many years, and this is unlikely to be accounted for simply by sedative withdrawal. Finally, given the difficulty of using ICD-9-CM codes to delineate recurrent events from carryover coding of prior events, we cannot conclude whether sepsis is associated just with a heightened risk of isolated seizures or also with recurrent seizures.
A growing body of evidence suggests that patients with sepsis are at risk of neurologic complications during their hospitalization. However, our findings support the hypothesis that sepsis is also associated with pathways that lead to long-lasting neurologic consequences, independent of clinically overt brain injury such as stroke or meningitis. These findings also raise the possibility that other acute systemic illnesses may be novel long-term seizure risk factors that have thus far gone unrecognized. Future investigations will be required to better understand the pathways underlying the long-term neurologic sequelae of sepsis and to determine their incidence, extent, and possible risk factors. Such knowledge may promote the development of brain-protective strategies that will help reduce the burden of this common disease.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Monica Chen for copyediting and clerical support.
GLOSSARY
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- ED
emergency department
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- ICU
intensive care unit
- IRR
incidence rate ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Michael E. Reznik made substantial contributions to the conception and design of the work, acquired, analyzed, and interpreted data for the work, drafted and revised the manuscript for intellectual content, and provided final approval of the version to be submitted. Alexander E. Merkler drafted and revised the manuscript for intellectual content, acquired, analyzed and interpreted data for the work, and provided final approval of the version to be submitted. Ali Mahta critically revised the manuscript for intellectual content and provided final approval of the version to be submitted. Santosh B. Murthy critically revised the manuscript for intellectual content and provided final approval of the version to be submitted. Jan Claassen critically revised the manuscript for intellectual content and provided final approval of the version to be submitted. Hooman Kamel acquired and analyzed the data, revised the work critically for intellectual content, provided study supervision, and provided final approval of the version to be submitted.
STUDY FUNDING
NIH grants K23NS082367 (H.K.) and the Michael Goldberg Research Fund (H.K.).
DISCLOSURE
M. Reznik, A. Merkler, and A. Mahta report no disclosures relevant to the manuscript. S. Murthy is supported by the American Brain Foundation and the American Academy of Neurology. J. Claassen reports no disclosures relevant to the manuscript. H. Kamel is supported by NIH grants K23NS082367, R01NS097443, and U01NS095869 as well as by the Michael Goldberg Research Fund. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour C, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (SEPSIS-3). JAMA 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweis R, Ortiz J, Biller J. Neurology of sepsis. Curr Neurol Neurosci Rep 2016;16:21. [DOI] [PubMed] [Google Scholar]
- 3.Widmann CN, Heneka MT. Long-term cerebral consequences of sepsis. Lancet Neurol 2014;13:630–636. [DOI] [PubMed] [Google Scholar]
- 4.Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol 2012;8:557–566. [DOI] [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Ely E, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010;304:1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semmler A, Widmann CN, Okulla T, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry 2013;84:62–69. [DOI] [PubMed] [Google Scholar]
- 7.Nishioku T, Dohgu S, Takata F, et al. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood–brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol 2009;29:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thibeault I, Laflamme N, Rivest S. Regulation of the gene encoding the monocyte chemoattractant protein 1 (MCP-1) in the mouse and rat brain in response to circulating LPS and proinflammatory cytokines. J Comp Neurol 2001;434:461–477. [DOI] [PubMed] [Google Scholar]
- 9.Semmler A, Hermann S, Mormann F, et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation 2008;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messaris E, Memos N, Chatzigianni E, et al. Time-dependent mitochondrial-mediated programmed neuronal cell death prolongs survival in sepsis. Crit Care Med 2004;32:1764–1770. [DOI] [PubMed] [Google Scholar]
- 11.d'Avila JdCP, Santiago APSA, Amâncio RT, Galina A, Oliveira MF, Bozza FA. Sepsis induces brain mitochondrial dysfunction. Crit Care Med 2008;36:1925–1932. [DOI] [PubMed] [Google Scholar]
- 12.Rosengarten B, Wolff S, Klatt S, Schermuly RT. Effects of inducible nitric oxide synthase inhibition or norepinephrine on the neurovascular coupling in an endotoxic rat shock model. Crit Care 2009;13:R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wazni OM, Martin DO, Marrouche NF, et al. Plasma B-type natriuretic peptide levels predict postoperative atrial fibrillation in patients undergoing cardiac surgery. Circulation 2004;110:124–127. [DOI] [PubMed] [Google Scholar]
- 14.Barrett M, Steiner C, Andrews R, Kassed C, Nagamine M. Methodological Issues When Studying Readmissions and Revisits Using Hospital Administrative Data. HCUP Methods Series Report # 2011-01; US Agency for Healthcare Research and Quality; 2011. Available at: https://www.hcup-us.ahrq.gov/reports/methods/2011_01.pdf. Accessed April 30, 2016. [Google Scholar]
- 15.White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005;111:1327–1331. [DOI] [PubMed] [Google Scholar]
- 16.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalizations. Crit Care Med 2005;33:2555–2562. [DOI] [PubMed] [Google Scholar]
- 17.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007;35:1244–1250. [DOI] [PubMed] [Google Scholar]
- 18.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. What is the best method for estimating the burden of severe sepsis in the United States? J Crit Care 2012;27:414.e1–414.e9. [DOI] [PubMed] [Google Scholar]
- 19.Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet 2009;373:1105–1110. [DOI] [PubMed] [Google Scholar]
- 20.Brown RD, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke 1996;27:373–380. [PubMed] [Google Scholar]
- 21.Christensen J, Vestergaard M, Pedersen MG, Pedersen CB, Olsen J, Sidenius P. Incidence and prevalence of epilepsy in Denmark. Epilepsy Res 2007;76:60–65. [DOI] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 23.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest 2014;146:1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kee VR, Gilchrist B, Granner MA, Sarrazin NR, Carnahan RM. A systematic review of validated methods for identifying seizures, convulsions, or epilepsy using administrative and claims data. Pharmacoepidemiol Drug Saf 2012;21(suppl 1):183–193. [DOI] [PubMed] [Google Scholar]
- 25.Pugh MJ, Van Cott AC, Cramer JA, et al. Trends in antiepileptic drug prescribing for older patients with new-onset epilepsy: 2000–2004. Neurology 2008;70:2171–2178. [DOI] [PubMed] [Google Scholar]
- 26.Hardie NA, Garrard J, Gross CR, et al. The validity of epilepsy or seizure documentation in nursing homes. Epilepsy Res 2007;74:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Coster C, Li B, Quan H. Comparison and validity of procedures coded with ICD-9-CM and ICD-10-CA/CCI. Med Care 2008;46:627–634. [DOI] [PubMed] [Google Scholar]
- 28.Kao LT, Sheu JJ, Lin HC, Tsai MC, Chung SD. Association between sepsis and dementia. J Clin Neurosci 2015;22:1430–1433. [DOI] [PubMed] [Google Scholar]
- 29.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369:1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit. Crit Care Med 2009;37:2051–2056. [DOI] [PubMed] [Google Scholar]
- 31.Gilmore EJ, Gaspard N, Choi HA, et al. Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med 2015;41:686–694. [DOI] [PubMed] [Google Scholar]
- 32.Gavvala JR, Schuele SU. New-onset seizure in adults and adolescents: a review. JAMA 2016;316:2657–2668. [DOI] [PubMed] [Google Scholar]
- 33.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993;34:453–458. [DOI] [PubMed] [Google Scholar]
- 34.Loiseau J, Loiseau P, Guyot M, Duche B, Dartigues JF, Aublet B. Survey of seizure disorders in the French southwest: I: incidence of epileptic syndromes. Epilepsia 1990;31:391–396. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald BK, Cockerell OC, Sander JWAS, Shorvon SD. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain 2000;123:665–676. [DOI] [PubMed] [Google Scholar]
- 36.Olafsson E, Ludvigsson P, Hesdorffer D, Kjartansson O, Hauser WA, Gudmundsson G. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurol 2005;4:627–634. [DOI] [PubMed] [Google Scholar]
- 37.Placencia M, Shorvon SD, Paredes V, et al. Epileptic seizures in an Andean region of Ecuador: incidence and prevalence and regional variation. Brain 1992;115:771–782. [DOI] [PubMed] [Google Scholar]
- 38.Jallon P, Smadja D, Cabre P, Le Mab G, Bazin M; EPIMART Group. EPIMART: prospective incidence study of epileptic seizures in newly referred patients in a French Caribbean Island (Martinique). Epilepsia 1999;40:1103–1109. [DOI] [PubMed] [Google Scholar]
- 39.Jallon P, Goumaz M, Haenggeli C, Morabia A. Incidence of first epileptic seizures in the Canton of Geneva, Switzerland. Epilepsia 1997;38:547–552. [DOI] [PubMed] [Google Scholar]
- 40.Lavados J, Germain L, Morales A, Campero M, Lavados P. A descriptive study of epilepsy in the district of El Salvador, Chile, 1984–1988. Acta Neurol Scand 1992;85:249–256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.