Abstract
Background
Brain energy metabolism is critical for supporting synaptic function and information processing. A growing body of evidence suggests abnormalities in brain bioenergetics in psychiatric disorders, including both bipolar disorder (BD) and schizophrenia. 31P magnetic resonance spectroscopy (31P-MRS) provides a non-invasive window into these processes in vivo. Using this approach, we previously showed that patients with bipolar disorder show normal ATP and phosphocreatine (PCr) levels at rest but cannot maintain normal ATP levels in the visual cortex during times of high energy demand (photic stimulation). Since ATP is replenished from PCr via the creatine kinase (CK) reaction, we have now measured the CK forward reaction rate constant (CK kf) in BD.
Methods
We studied 20 patients experiencing a first episode of BD and 28 healthy controls at 4 Tesla and quantified CK kf using 31P Magnetization Transfer MRS (31P-MT-MRS) as previously described.
Results
We found a significant reduction in CK kf in the BD group (F=4.692, p=0.036), while brain ATP and PCr concentrations, as well as brain parenchymal pH were normal.
Conclusions
These results pinpoint a specific molecular mechanism underlying our previous observation of an inability to replenish brain ATP during times of high energy demand in BD.
Keywords: bioenergetics, MRS, mitochondria, ATP, mania, imaging
INTRODUCTION
Energy metabolism and related mitochondrial functions are critical for healthy brain development and sustaining fundamental mechanisms such as synaptic neurotransmission, maintenance of ion gradients, and intracellular signaling. Consequently, abnormalities in these processes in major psychiatric disorders such as schizophrenia (SZ) and bipolar disorder (BD) have attracted growing interest. These include abnormalities in the metabolism of energy-related molecules, dysfunctional oxidative phosphorylation, reduced expression of mitochondria related genes, and altered morphology and cellular location of mitochondria (1–4).
Adenosine triphosphate (ATP) is the brain’s primary energy currency. Although ATP is largely generated de novo by oxidative phosphorylation in mitochondria, its high energy phosphate (HEP) moiety is rapidly transferred to creatine (Cr) to generate phosphocreatine (PCr) in a reaction catalyzed by the reversible enzyme CK. PCr diffuses throughout the cytosol and is used to regenerate ATP locally via the CK reaction as required for cellular reactions. Cells maintain ATP levels within a narrow range but PCr levels are allowed to fluctuate as an energy storage buffer; the CK reaction is the main source of ATP generation in the cytosol (5, 6).
We recently investigated HEP molecule dynamics in BD at baseline and during brain activation using a photic stimulation paradigm (7). Although ATP and PCr levels in the occipital lobe were normal at baseline, BD patients showed an inability to replenish ATP from PCr during stimulation. This resulted in reduced ATP levels but no change in PCr during stimulation, in contrast to healthy individuals who showed the predicted reduction in PCr with no change in ATP. This study suggested a CK abnormality in BD potentially resulting in reduced ATP availability in the brain during times of high demand; but CK reaction rates have not yet been measured directly in this condition. This can be done in vivo using 31P magnetization transfer magnetic resonance spectroscopy (31P-MT-MRS) (8). With this method, we recently found a significant reduction in the CK reaction rate in the frontal lobe of SZ patients compared to healthy individuals (9). Studies such as these and others (10, 11) examining specific bioenergetic processes in psychiatric patients in vivo are promising because they may allow us to develop new treatment strategies targeting these specific processes, as well as providing biomarkers to monitor treatment response and disease progression.
In the current study, we used 31P-MT-MRS to investigate the CK reaction rate catalyzing the flow of HEP from PCr to ATP (abbreviated CK kf) in patients experiencing a first manic episode (first-episode BD) to complement our prior observations (7). This is the first study that directly investigates a specific enzymatic reaction in this disorder in vivo. Based on our previous study, we hypothesized that the CK reaction rate would be reduced in this patient group compared to healthy individuals, reflecting reduced ATP availability at times of increased energy demand.
METHODS
Participants
Participants included 20 first episode patients with a DSM-IV diagnosis of BD-I with psychotic features and 28 age and sex-matched healthy controls (HC). Patients were recruited from McLean OnTrack, a first-episode psychosis treatment service at McLean Hospital (12). All except 3 patients in the BD group were taking psychotropic medication at the time of the scan. Two healthy control participants and 3 patients overlapped with the sample in our previous publication (7). First-episode psychosis at the time of entry to the clinic was defined as having an onset of overt psychotic symptoms within the past 1 year, no more than one lifetime psychiatric hospitalization and less than 24 weeks of psychotropic medication use. All patients were between the ages of 18 and 30, had no significant past or current neurological or medical disorders, intellectual disability, a history of head trauma with loss of consciousness, or had received ECT within the last 3 months. We also excluded individuals using supplements that affect HEP levels, such as creatine. The HC met the same criteria and in addition had no personal or first-degree relative history of any psychiatric disorders.
This study was approved by the McLean Hospital IRB. All participants provided written informed consent. Diagnostic assessments were carried out using the Structured Clinical Interview for DSM-IV for patients and healthy controls. The Positive and Negative Symptom Scale (PANSS), the Young Mania Rating Scale (YMRS), and the Montgomery-Asberg Depression Rating Scale (MADRS) were used for clinical measures. The sociodemographic and clinical characteristics of participants are presented in Table 1.
Table 1.
Demographic and clinical characteristics of the participants
| Characteristic | BD patients (n=20) |
Healthy controls (n=28) |
|---|---|---|
| Age (range) | 22.3 (18–29) | 23.4 (18–28) |
| Sex (M/F)a | 13/7 | 9/19 |
| Educationa, b | 4.9 ± 1.6 | 5.9 ± 1.6 |
| BMI | 23.4 ± 4.3 | 23.7 ± 3.9 |
| PANSS | ||
| Positive | 9.6 ± 6.9 | – |
| Negative | 9.4 ± 4.8 | – |
| General | 21.9 ± 5.9 | – |
| YMRS | 5.8 ± 6.9 | – |
| MADRS | 6.9 ± 7.6 | – |
| MCAS | 48.5 ± 10.4 | – |
| NAART | 113.8 ± 6.8 | – |
| CPZ equivalent | 119.2 ±121.3 | – |
| Lithium | 11 (55 %) | – |
| Valproate | 2 (10 %) | – |
| Antipsychotic | 13 (65 %) | – |
| Substance use disorder | 15 (75 %) | – |
p<0.05
Range is 3 (high school graduate), 4 (some college), 5 (2-year college graduate), 6 (4-year college graduate), 7 (some graduate or professional school), and 8 (completed graduate or professional school).
All data are expressed with mean ± SD.
Abbreviations: BD, bipolar disorder; BMI, Body Mass Index; CPZ, chlorpromazine; MADRS, Montgomery Asberg Depression Rating Scale; MCAS, Multnomah Community Ability Scale; NAART, North American Adult Reading Test; PANSS, Positive and Negative Syndrome Scale; YMRS, Young Mania Rating Scale
Magnetic Resonance Imaging and In-Vivo 31P-MT-MRS Experiments
All methodological details are identical to those in our previous publication (9). Briefly, the 31P MT MRS study-related acquisitions were conducted using a 4 Tesla whole-body imaging system (Unity/Inova; Varian NMR Instruments) using a specially designed half-helmet head coil with dual tuned proton and phosphorus frequency channels placed on the forehead. Following rapid 2-dimensional gradient-recalled echo images in 3 dimensions, After global shimming of unsuppressed water signal (water linewidth of 24 Hz or less) and high contrast T2-weighted sagittal and axial images, we positioned the MRS voxels in the frontal lobe. Localized shimming with a voxel of 6 × 6 × 4 cm3 on the frontal lobe (Figure 1) was performed for 31P-MT-MRS. The 31P signal was acquired with outer-volume saturation (13). The 31P-MT pulse sequence and experimental design have been described previously (14, 15). A pulse train with varied RF pulse amplitudes according to the B1-insensitive train to obliterate signal (BISTRO) scheme (16) was used to saturate selectively the resonance peak of γ-ATP for measurement of the CK reaction rate constant. Saturation time (0 to 12.28s) was controlled by varying the cycling number of the BISTRO pulse train. A 200-μs hard RF pulse was used to excite the phosphate spins and its flip angle (nominal 90°) was adjusted to achieve optimal NMR signal. In vivo 31P spectra were acquired using the following parameters: 5000Hz spectral width, 1024 complex data points, 32 signal averages. All in vivo 31P spectra were acquired with a 14-s repetition time (TR) in approximately fully relaxed condition. In order to avoid any confounds from machine instability or subject movements, 31P-MT-MRS was acquired in interleaved fashion, i.e. with varying saturation times over a single acquisition, then repeat. The specific absorption rate (SAR) was maintained below FDA limits. The total measurement time (MRI and MRS) was ≤ 75 min.
Figure 1.
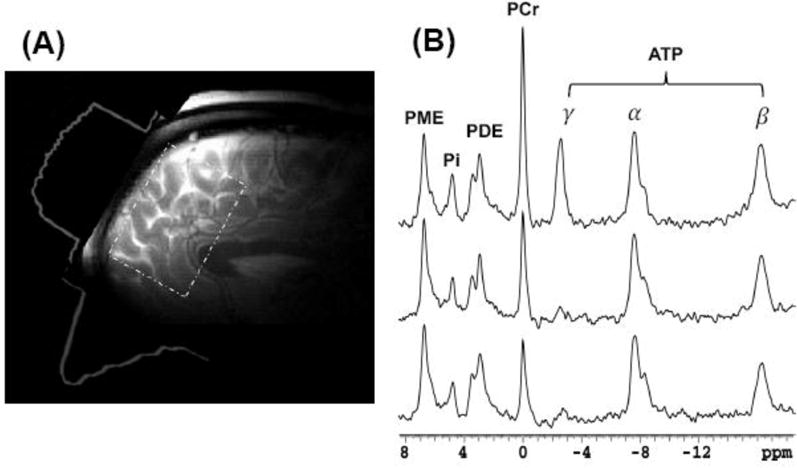
(a) 31P surface coil placed over forehead and its sensitivity profile (6 × 6 × 4 cm3).
(b) In vivo 31P spectra obtained from a representative healthy volunteer in the absence (upper tracing) and presence of γ-ATP saturation (middle and bottom tracings, with saturation time of 6.6s and 12.3s, respectively). The reduction of PCr resonance magnitude during γ- ATP saturation indicates transfer of high energy phosphates from PCr to ATP catalyzed by CK enzyme.
Forward Rate Constant and Flux of CK Reaction
The chemical exchange reaction among PCr and ATP as well as the relative chemical reaction parameters are described as follows (17, 18):
| (1) |
| (2) |
where kf (s−1) is the pseudo first-order forward rate constant of the CK reaction, which converts PCr to ATP. In Equation 2, k is a parameter related to the CK enzyme activity, partially modulated by the concentration of the CK enzyme or its 3D structure. Specifically, kf can be determined by the experiments using progressive saturation on the γ-ATP resonance, where the magnetizations of PCr is governed by Equation 3 (19):
| (3) |
In the above equation, Ms and M0 are the magnetization of PCr at saturation time (t) and Boltzmann thermal equilibrium condition, respectively. T1 is the intrinsic spin-lattice relaxation time of PCr. Therefore, the kf of the CK reaction and the T1 of PCr can be determined by fitting the experimental data to a single exponential decay, according to Equation 3. Seven saturation time points (0, 0.48, 1.89, 3.78, 6.61, 8.50, and 12.28 seconds) were applied in the present study.
Lastly, the chemical reaction flux (F) is calculated by:
where [M] is the metabolite concentration (μmol/ml) of PCr, determined via the PCr/γ-ATP ratio assuming [ATP]=3.0 mM(20). The chemical reaction fluxes were converted to the well-accepted units of μmol/g/min using an assumed brain tissue density of 1.1g/ml (14, 15).
31P-MRS Data Processing
The initial 4 FID points were truncated for data processing. Eight resonance peaks (PME: phosphomonoester modeled as a single peak; Pi; PDE: phosphodiester, modeled with two components glycerophosphocholine and glycerophosphoethanolamine; PCr; and three adenosine triphosphates: α-, β-, γ-ATP) were included in the basis set. Detailed spectrum processing has been described previously (14, 15). Since we acquired spectra in the absence of saturation at approximately full relaxation, we were able to quantify metabolites concentration ratios of phosphate compounds, expressed using an internal reference of γ-ATP. We also present the data with beta-ATP ratios in Supplemental Table S1.
Statistical Approach
All analyses were carried out using SPSS (V.17). Two-sample t-tests and chi-square tests were used to compare demographic characteristics and linewidth of the PCr resonance for 31P-MRS data (as a data quality measure) across groups.
Next, we carried out a series of analyses using the Pearson’s R correlation coefficient to examine the relationship between 31P-MRS measures (steady-state metabolite ratios, pH, and forward rate constant) on the one hand and age and BMI on the other for the full dataset, and the same 31P-MRS measures on the one hand and duration of illness, NAART score, CPZ equivalents, PANSS, YMRS, and MADRS on the other for the BD group. These were exploratory analyses since we have little prior knowledge to focus our analysis in this first implementation of a novel approach in a clinical study. We viewed these correlation analyses as providing leads for future studies to pursue systematically. Given the large number of comparisons in this analysis, we considered an R value >0.5 (generally considered a moderately strong association) as meaningful.
Our primary outcome measure was CK kf. We tested group differences in this dependent variable using an ANCOVA with diagnosis as a between-subjects factor, and education and gender as covariates because these variables were different between groups. We next carried out similar secondary ANCOVA analyses with parenchymal pH and with the ratios Pi/γ-ATP, PCr/γ-ATP, PME/γ-ATP and PDE/γ-ATP as the dependent variables.
We also explored the association of our primary outcome variable CK kf with factors related to psychiatric history, including presence of alcohol use disorders, substance use disorders, any anxiety disorder, number of mood episodes, and number of depressive episodes, using regression models.
A value of P=0.05 was used as the threshold for statistical significance for our primary analysis. Threshold for the secondary ANCOVAs was set at p = 0.01 (0.05/5). All data are reported as mean +/− standard deviation.
RESULTS
Demographic Variables
There was no difference in age, BMI, or handedness between groups. Level of education as ordinal categories in SCID (p = 0.035) and the proportion of females (p = 0.039) were higher in the healthy control group (Table 1). Gender was not significantly associated with any spectroscopic measures (F=1.066, p=0.391).
CK kf and Flux
The mean (SD) quality control measure of linewidth (with 10-Hz line broadening applied) and the signal noise ratio (SNR) of the PCr resonance did not differ for the first acquired spectra between the HC and BD groups [Linewidth: HC = 18.9 (2.6) and BD = 19.9 (2.2), p = 0.410; SNR: HC = 43.4 (17.9) and BD = 40.2 (23.3), p = 0.619].
Data from representative 31P-MT-MRS experiments are shown in Figure 1 and the results are presented in Table 2. The BD group showed a statistically significant 13% reduction in CK kf (F= 4.999, p = 0.031). We also calculated the flux (F) through this reaction, although this was not our primary measure. This parameter was decreased by 12% in BD (F=4.402, p =0.042).
Table 2.
Results of 31P Magnetization Transfer Spectroscopy Measurements in the Human Frontal Lobe of Patients and Healthy Control Subjects
| Measure | BD (n=20) | HC (n=28) | F/t | p |
|---|---|---|---|---|
| Metabolite ratio | ||||
| Phosphocreatine to γ-ATP | 1.34 (0.16) | 1.31 (0.16) | 0.151 | 0.700 |
| Inorganic phosphate to γ-ATP | 0.38 (0.08) | 0.39 (0.07) | 0.044 | 0.835 |
| Phosphodiester to γ-ATP | 0.76 (0.13) | 0.86 (0.19) | 4.500 | 0.038 |
| Phosphomonoester to γ-ATP | 1.09 (0.14) | 1.06 (0.13) | 0.682 | 0.413 |
| Intracellular pH | 7.02 (0.02) | 7.02 (0.02) | 0.097 | 0.757 |
| Rate constant kf of creatine kinase, s−1 | 0.21 (0.05) | 0.24 (0.04) | 4.990 | 0.031 |
| Flux of creatine kinase, μmol/g/min | 46.06 (12.8) | 51.87 (12.62) | 4.402 | 0.042 |
| Magnesium ion concentration, mmol/L | 0.16 (0.03) | 0.16 (0.03) | 0.154 | 0.698 |
All measures presented as mean (SD)
Other 31P-MRS Measures
There was no significant difference in parenchymal pH between BD group compared with the HC group. (F = 0.097, p = 0.757) (Table 2).
Metabolite ratios are provided in Table 2. We have reported metabolite-level results using γ-ATP as an internal reference to control for participant-specific sources of variance. No between-group differences survived multiple comparisons correction (p<0.010) in any metabolite ratio although a numerical reduction in PDE in the BD group approached significance (p=0.038). In addition, magnesium ion concentrations, deduced from chemical shifts of β-ATP resonance, were similar in the BD and HC groups (Table 2).
Additional Analyses
Compositions of gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) in our interested MRS region were not different between groups: GM (HC=0.418, BD=0.421; p=0.839), WM (HC=0.278, BD=0.285; p=0.176), CSF (HC=0.211, BD=0.220; p=0.349). When relative ratios of GM/WM/CSF were included as covariates, CK kf was still significantly different between groups (F=6.023, p=0.018). Results of the comparisons for phosphorus metabolites levels were also not changed (now shown).
To assess the reliability of our spectroscopic measures, we scanned 6 healthy control participants twice within one-week period. Coefficient of variation (CVs), calculated as the ratio of the SD to the mean, was used as the reliability measure. The CVs for CK kf, PCr/γ-ATP, Pi/γ-ATP, PDE/γ-ATP and PME/γ-ATP were 10.2%, 7.8%, 14.5%, 6.9%, 9.7% respectively, suggesting a good test-retest reliability.
No significant correlations (Pearson’s R>0.500) were found between spectroscopic parameters and demographic variables (in either group) or between spectroscopic parameters and clinical variables (for the patient group). None of the factors related to psychiatric history carried a significant association with CK kf: Alcohol use disorders (F=0.326, p=0.577), substance use disorders (F=0.278, p=0.606), anxiety disorders (F=0.221, p=0.646), number of mood episodes (F=0.480, p=0.500), number of depressive episodes (F=3.553, p=0.080).
DISCUSSION
In this study, we measured the forward reaction rate constant of the CK enzyme (CK kf) in a group of patients with first episode BD and healthy controls at 4 Tesla using 31P-MT-MRS. Our primary finding is a significant reduction in CK kf in BD patients in the absence of concentration abnormalities in ATP and PCr. This pattern complements our previous finding of normal baseline ATP and PCr concentrations at baseline but an inability to replenish brain ATP concentrations during times of high energy demand in BD (7). The picture emerging from our 31P-MRS studies is that individuals with BD can maintain normal brain concentrations of HEP metabolites at rest but there is an underlying abnormality in the mechanism that generates new ATP, which can be unmasked when energy demand is increased. This is consistent with the pathophysiology of BD, since patients with this condition can experience periods of euthymia and normal community functioning but are vulnerable to the disruptive effects of sleep deprivation, psychosocial stress, drugs of abuse, and other factors that significantly alter brain activity.
Although we have observed significant bioenergetics abnormalities in BD using 31P-MT MRS, not all aspects of brain metabolism are abnormal in this condition. For example, we found normal brain parenchymal pH in this study. This finding in first episode BD is consistent with our report in chronic BD (7) although we did find acidic pH in a cohort of patients with chronic schizophrenia (9). Others have reported pH reductions in BD (21) as well as elevated lactic acid concentrations in medication-free patients with BD (22), suggesting that these abnormalities may be associated with BD depending on characteristics of the patient population and psychotropic medication status. Brain pH is of interest because acidity largely reflects lactic acid build-up potentially as a result of a shift in relative energy production from oxidative phosphorylation to glycolysis.
One important feature of the current study has to do with the clinical characteristics of the patient population. We studied patients recovering from a first psychotic manic episode. These patients therefore have already been diagnosed with BD, but they have not been exposed to the potential toxic effects of chronic psychiatric illness or long-term psychotropic medications. The mean age of the patient group at 22 reflects the early phase of illness. Therefore, our findings are more likely to be related to pathophysiologic factors and not to chronic illness. In addition, the symptom scale data in Table 1 indicate that our patient cohort was not acutely ill at the time of study, with low YMRS and PANSS scores. This suggests that the abnormalities we observe are not related to acute symptomatology (i.e. state-related) but rather to the trait of BD.
Among the other findings in this study, we note that the concentration of PDEs was lower in the patient group compared to controls. This reduction was nominally statistically significant, although the finding does not survive our multiple comparisons correction. This finding is similar in kind to what we reported in our chronic SZ study (9). In BD, studies that reported on PDE found no change in frontal or temporal lobes (23–26), except one study that found an increase (27). PME on the other hand was generally reduced (reviewed in (28)). None of these studies were performed in first episode patients. Clinical and demographic differences between subjects in different studies or sampling error in these studies which were of modest size may explain the discrepant findings.
This study has certain limitations. As with most brain imaging studies, the sample size is modest. Also, the potential for medication effects is important, since psychotropic medications are known to have effects on brain metabolism (e.g.(29). In this study we recruited patients who had minimal exposure to medication but we cannot rule out medication induced effects on brain metabolism in early stages of pharmacotherapy. To address this concern, we have carried out a correlation analysis between mean daily dose of CPZ equivalent and our brain imaging measures and have found no evidence of a relationship. In addition, the three individuals in the BD group who were not taking psychotropic medication at scan time showed a CK kf reduction similar to that seen in the overall BD group. Finally, we have seen evidence in other studies that the medication effects may be ameliorative not disruptive to brain metabolism (30). Taken together, we do not see evidence that our findings are driven by medication effects as opposed to disease-specific effects, although this possibility cannot be ruled out.
The group differences in education and gender are potential confounds that could have affected our results directly or by mediators such as body mass index. However, we included these variables as covariates in all our analyses, and body mass index was not different between groups. All patient participants had experienced a manic episode with psychotic features. While the association of psychosis with our findings is not clear, psychotic symptoms are experienced by majority of BD patients during manic episodes (31) and therefore our sample is representative of the treatment-seeking BD population.
We used γ-ATP as internal reference in metabolite ratio calculations. Therefore, differences in γ-ATP between groups could lead to miscalculations in these measures. We have not quantified ATP absolute levels, due to technical challenges, so we cannot address this concern. Another limitation is that in this study we collected 31P-MT-MRS data at baseline in the PFC and not during brain stimulation in the occipital cortex. Our original observation of a difficulty replenishing ATP was made in the context of normal baseline findings and an abnormal response to photic stimulation (7). Therefore, it would be desirable to collect enzymatic reaction rate data during a stimulation paradigm. This conventional approach is technically challenging, however, because the duration of 31P-MT MRS data collection (30 minutes or longer) lasts much longer than the time subjects can tolerate brain stimulation paradigms (12 minutes in our earlier study) and MRS sensitivity with the smaller voxel size on the occipital lobe tends to be lower than that of prefrontal lobe. Therefore, we elected to collect CK kf data at baseline in the PFC when highest quality data could be collected. In future studies, novel 31P-MT MRS approaches which afford shorter acquisition times may help address this issue (15, 32). Our finding of reduced CK kf in BD even at baseline is consistent with our prior findings because a CK kf abnormality may not manifest itself as a concentration abnormality during steady state when sufficient ATP is being generated even through a slower-than normal reaction. It should be noted that BD, and other mood and psychotic disorders, are predominantly determined by genetic factors, and these factors may produce similar physiologic deficits throughout the brain and even in peripheral tissues, as noted in previous studies.
In summary, we report an enzymatic reaction rate abnormality in first episode BD in the molecular machinery, the CK enzymatic reaction, used to support brain activity and information processing. 31P MRS cannot differentiate between the cytosolic and mitochondrial compartments but capture the activity of overall CK in the forward direction. There is evidence for decreased transcription of both brain and mitochondrial isoforms of CK in BD which can explain the reduction in kf (4), however alteration in translational or post-translational modification (33) cannot be excluded. Our finding builds upon our previous report (7), and specifically pinpoints the CK enzyme as a molecular bottleneck that leads to reduced availability of ATP in support of brain energy metabolism. This reduced ATP availability may have implications for the maintenance or disruption of critical brain functions such as synaptic activity and information processing in bipolar disorder (34).
Supplementary Material
Acknowledgments
This work was supported by K24MH104449 (DO) and the Program for Neuropsychiatric Research at McLean Hospital (BMC). The authors thank Samira Pingali for assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2011;29:311–324. doi: 10.1016/j.ijdevneu.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cataldo AM, McPhie DL, Lange NT, Punzell S, Elmiligy S, Ye NZ, et al. Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. The American journal of pathology. 2010;177:575–585. doi: 10.2353/ajpath.2010.081068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman O, Ben-Shachar D. Mitochondrial Oxidative Phosphorylation System (OXPHOS) Deficits in Schizophrenia: Possible Interactions with Cellular Processes. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2016;61:457–469. doi: 10.1177/0706743716648290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald ML, Naydenov A, Chu M, Matzilevich D, Konradi C. Decrease in creatine kinase messenger RNA expression in the hippocampus and dorsolateral prefrontal cortex in bipolar disorder. Bipolar Disord. 2006;8:255–264. doi: 10.1111/j.1399-5618.2006.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemp GJ. Non-invasive methods for studying brain energy metabolism: what they show and what it means. Developmental neuroscience. 2000;22:418–428. doi: 10.1159/000017471. [DOI] [PubMed] [Google Scholar]
- 6.Sahlin K, Harris RC. The creatine kinase reaction: a simple reaction with functional complexity. Amino acids. 2011;40:1363–1367. doi: 10.1007/s00726-011-0856-8. [DOI] [PubMed] [Google Scholar]
- 7.Yuksel C, Du F, Ravichandran C, Goldbach JR, Thida T, Lin P, et al. Abnormal high-energy phosphate molecule metabolism during regional brain activation in patients with bipolar disorder. Mol Psychiatry. 2015;20:1079–1084. doi: 10.1038/mp.2015.13. [DOI] [PubMed] [Google Scholar]
- 8.Du F, Cooper A, Lukas SE, Cohen BM, Ongur D. Creatine kinase and ATP synthase reaction rates in human frontal lobe measured by (3)(1)P magnetization transfer spectroscopy at 4T. Magn Reson Imaging. 2013;31:102–108. doi: 10.1016/j.mri.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du F, Cooper AJ, Thida T, Sehovic S, Lukas SE, Cohen BM, et al. In vivo evidence for cerebral bioenergetic abnormalities in schizophrenia measured using 31P magnetization transfer spectroscopy. JAMA Psychiatry. 2014;71:19–27. doi: 10.1001/jamapsychiatry.2013.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley J, DelBello MP, Weber WA, Adler CM, Strakowski SM, Lee JH. Tissue-dependent cerebral energy metabolism in adolescents with bipolar disorder. Journal of affective disorders. 2016;191:248–255. doi: 10.1016/j.jad.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 11.Nenadic I, Dietzek M, Langbein K, Rzanny R, Gussew A, Reichenbach JR, et al. Effects of olanzapine on 31P MRS metabolic markers in schizophrenia. Hum Psychopharmacol. 2013;28:91–93. doi: 10.1002/hup.2274. [DOI] [PubMed] [Google Scholar]
- 12.Shinn AK, Bolton KW, Karmacharya R, Lewandowski KE, Yuksel C, Baker JT, et al. McLean OnTrack: a transdiagnostic program for early intervention in first-episode psychosis. Early Interv Psychiatry. 2015 doi: 10.1111/eip.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaumeil MM, Valette J, Guillermier M, Brouillet E, Boumezbeur F, Herard AS, et al. Multimodal neuroimaging provides a highly consistent picture of energy metabolism, validating 31P MRS for measuring brain ATP synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3988–3993. doi: 10.1073/pnas.0806516106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, et al. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A. 2008;105:6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magnetic resonance in medicine. 2007;57:103–114. doi: 10.1002/mrm.21107. [DOI] [PubMed] [Google Scholar]
- 16.de Graaf RA, Luo Y, Garwood M, Nicolay K. B1-insensitive, single-shot localization and water suppression. Journal of magnetic resonance Series B. 1996;113:35–45. doi: 10.1006/jmrb.1996.0152. [DOI] [PubMed] [Google Scholar]
- 17.Petroff OA, Prichard JW, Behar KL, Alger JR, den Hollander JA, Shulman RG. Cerebral intracellular pH by 31P nuclear magnetic resonance spectroscopy. Neurology. 1985;35:781–788. doi: 10.1212/wnl.35.6.781. [DOI] [PubMed] [Google Scholar]
- 18.Degani H, Alger JR, Shulman RG, Petroff OA, Prichard JW. 31P magnetization transfer studies of creatine kinase kinetics in living rabbit brain. Magn Reson Med. 1987;5:1–12. doi: 10.1002/mrm.1910050102. [DOI] [PubMed] [Google Scholar]
- 19.Degani H, Laughlin M, Campbell S, Shulman RG. Kinetics of creatine kinase in heart: a 31P NMR saturation- and inversion-transfer study. Biochemistry. 1985;24:5510–5516. doi: 10.1021/bi00341a035. [DOI] [PubMed] [Google Scholar]
- 20.Hetherington HP, Spencer DD, Vaughan JT, Pan JW. Quantitative (31)P spectroscopic imaging of human brain at 4 Tesla: assessment of gray and white matter differences of phosphocreatine and ATP. Magnetic resonance in medicine. 2001;45:46–52. doi: 10.1002/1522-2594(200101)45:1<46::aid-mrm1008>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 21.Kato T, Murashita J, Kamiya A, Shioiri T, Kato N, Inubushi T. Decreased brain intracellular pH measured by 31P-MRS in bipolar disorder: a confirmation in drug-free patients and correlation with white matter hyperintensity. Eur Arch Psychiatry Clin Neurosci. 1998;248:301–306. doi: 10.1007/s004060050054. [DOI] [PubMed] [Google Scholar]
- 22.Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- 23.Shi XF, Carlson PJ, Sung YH, Fiedler KK, Forrest LN, Hellem TL, et al. Decreased brain PME/PDE ratio in bipolar disorder: a preliminary (31) P magnetic resonance spectroscopy study. Bipolar disorders. 2015;17:743–752. doi: 10.1111/bdi.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato T, Takahashi S, Shioiri T, Inubushi T. Alterations in brain phosphorous metabolism in bipolar disorder detected by in vivo 31P and 7Li magnetic resonance spectroscopy. Journal of affective disorders. 1993;27:53–59. doi: 10.1016/0165-0327(93)90097-4. [DOI] [PubMed] [Google Scholar]
- 25.Kato T, Takahashi S, Shioiri T, Inubushi T. Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. Journal of affective disorders. 1992;26:223–230. doi: 10.1016/0165-0327(92)90099-r. [DOI] [PubMed] [Google Scholar]
- 26.Deicken RF, Weiner MW, Fein G. Decreased temporal lobe phosphomonoesters in bipolar disorder. Journal of affective disorders. 1995;33:195–199. doi: 10.1016/0165-0327(94)00089-r. [DOI] [PubMed] [Google Scholar]
- 27.Deicken RF, Fein G, Weiner MW. Abnormal frontal lobe phosphorous metabolism in bipolar disorder. The American journal of psychiatry. 1995;152:915–918. doi: 10.1176/ajp.152.6.915. [DOI] [PubMed] [Google Scholar]
- 28.Yildiz A, Sachs GS, Dorer DJ, Renshaw PF. 31P Nuclear magnetic resonance spectroscopy findings in bipolar illness: a meta-analysis. Psychiatry research. 2001;106:181–191. doi: 10.1016/s0925-4927(01)00082-8. [DOI] [PubMed] [Google Scholar]
- 29.Contreras-Shannon V, Heart DL, Paredes RM, Navaira E, Catano G, Maffi SK, et al. Clozapine-induced mitochondria alterations and inflammation in brain and insulin-responsive cells. PLoS One. 2013;8:e59012. doi: 10.1371/journal.pone.0059012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SY, Cohen BM, Chen X, Lukas SE, Shinn AK, Yuksel AC, et al. Redox Dysregulation in Schizophrenia Revealed by in vivo NAD+/NADH Measurement. Schizophr Bull. 2016 doi: 10.1093/schbul/sbw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunayevich E, Keck PE., Jr Prevalence and description of psychotic features in bipolar mania. Current psychiatry reports. 2000;2:286–290. doi: 10.1007/s11920-000-0069-4. [DOI] [PubMed] [Google Scholar]
- 32.Tusek Jelenc M, Chmelik M, Bogner W, Krssak M, Trattnig S, Valkovic L. Feasibility and repeatability of localized (31) P-MRS four-angle saturation transfer (FAST) of the human gastrocnemius muscle using a surface coil at 7 T. NMR Biomed. 2016;29:57–65. doi: 10.1002/nbm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin G, Liu Y, MacLeod KM. Regulation of muscle creatine kinase by phosphorylation in normal and diabetic hearts. Cellular and molecular life sciences: CMLS. 2009;66:135–144. doi: 10.1007/s00018-008-8575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreazza AC, Wang JF, Salmasi F, Shao L, Young LT. Specific subcellular changes in oxidative stress in prefrontal cortex from patients with bipolar disorder. J Neurochem. 2013 doi: 10.1111/jnc.12316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


