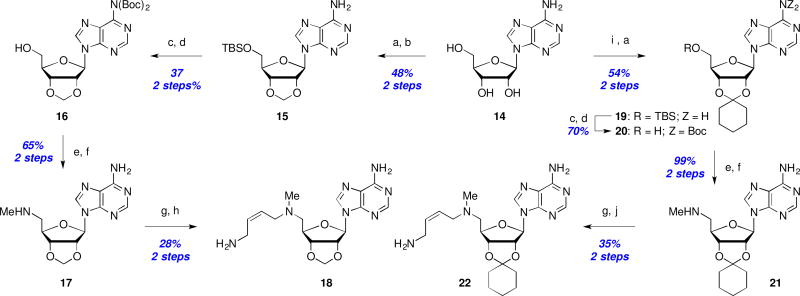
Scheme 3.
Synthesis of ribose-ketal analogs: Reagents and conditions: (a) TBSCl, imid., DMF, rt, 2–24 h; (b) CH2Br2, NaOH, TBAB, CH2Cl2/H2O, 40 °C, 72 h; (c) Boc2O, DMAP, DMF, 18 h; (d) TBAF, THF, 1–18 h; (e) MsCl, Et3N, CH2Cl2, 1 h; (f) MeNH2/EtOH, THF, 50 °C, 18 h; (g) 13, Et3N, NaI, MeCN, rt, 3–18 h; (h) 1M H2SO4, MeOH, rt, 18 h; (i) cyclohexanone, TFA, rt; (j) TFA, CH2Cl2, rt, 1 h.