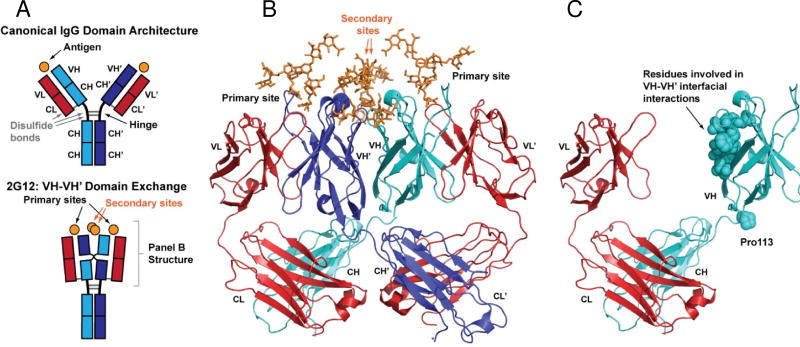
Fig. 1. Domain-exchange architecture of 2G12.
(A) Schematic of domain architecture for canonical IgG molecules (top) and 2G12 (bottom). Canonical IgG architecture results in two antigen binding sites at the interface of light and heavy chain variable domains (VL-VH and VL’-VH’). The VH-VH’ domain exchange in 2G12 creates an extended antigen binding surface that contains up to two additional binding sites at the VH-VH’ interface. (B) Crystal structure of the 2G12 Fab dimer in complex with Man9GlcNAc2 (orange) reported by Calarese et al. (PDB ID 1OP5)6. Domain colors as shown in panel A. (C) Fab monomer of 2G12 showing features that stabilize the domain exchange.