Abstract
Background
Stress constitutes a risk factor across several psychiatric disorders. Moreover, females are more susceptible to stress-related disorders, such as depression, than males. Although dopamine system underactivation is implicated in the pathophysiology of depression, little is known about the female dopamine system at baseline and post-stress.
Methods
The effects of chronic mild stress were examined on ventral tegmental area dopamine neuron activity and forced swim test immobility by comparing male and female rats. The impact of a single dose of the rapid antidepressant ketamine (10 mg/kg, i.p.) on forced swim test immobility and ventral tegmental area function was then tested.
Results
Baseline ventral tegmental area dopamine activity was comparable in both sexes. At baseline, females exhibited roughly double the forced swim test immobility duration than males, which corresponded to ~50% decrease in ventral tegmental area dopamine population activity compared with similarly treated (i.e., post-forced swim test) males. Following chronic mild stress, there was greater immobility duration in both sexes and reduced ventral tegmental area dopamine neuron activity by approximately 50% in males and nearly 75% in females. Ketamine restored behavior and post-forced swim test ventral tegmental area dopamine activity for up to 7 days in females as well as in both male and female chronic mild stress-exposed rats.
Conclusions
These data suggest increased female susceptibility to depression-like phenotypes (i.e., greater immobility, ventral tegmental area hypofunction) is associated with higher dopamine system sensitivity to both acute and repeated stress relative to males. Understanding the neural underpinnings of sex differences in stress vulnerability will provide insight into mechanisms of disease and optimizing therapeutic approaches in both sexes.
Keywords: sex differences, stress, dopamine, depression, ketamine
Significance Statement
Depression, the most prevalent psychiatric disorder, is twice as common in females. Depression involves downregulation of the dopamine (DA) system. However, little is known about the female dopamine system at baseline and how it reacts to stressors. We employed a rodent model of chronic mild stress (CMS), which produces neurobehavioral adaptations relevant to depression, and utilized behavioral and electrophysiological techniques to compare the effects on male and female rats. Moreover, we assessed the antidepressant-like effects of an acute dose of ketamine on immobility behavior and VTA activity. Although DA system function was similar in both sexes at baseline, swim stress and CMS caused a greater attenuation of the DA system in females. Ketamine reduced stress-induced behavioral abnormalities and restored normal DA system function in both sexes. These findings provide insight into sex differences in stress-induced neurobehavioral adaptations and contribute to our understanding of treatment for stress-related disorders implicating DA malfunction.
Introduction
Uncontrollable stress exposure is tightly linked to the development of depression (Lloyd, 1980; Kendler et al., 1999; Holsboer, 2001), and women in particular are more susceptible than men to stress-related disorders and twice as likely to experience depression (Kessler, 2003; Parker and Brotchie, 2010; Seney and Sibille, 2014). Depression has been linked to alterations in the mesolimbic dopamine (DA) system (Nestler and Carlezon, 2006; Yadid and Friedman, 2008), which originates in the ventral tegmental area (VTA) (Kalivas, 1993; Marinelli and McCutcheon, 2014), and is characterized by DA system downregulation (Martin-Soelch, 2009; Belujon and Grace, 2014; Chang and Grace, 2014). Indeed, a causal link between stress-induced depression-related behaviors and DA system hypofunction has been established using rodent models (Chaudhury et al., 2013; Tye et al., 2013). Yet little is known about the female DA system at baseline and post-stress due to an overreliance on male subjects, animals, and cells in biomedical research (Beery and Zucker, 2011; Clayton and Collins, 2014).
Chronic stress is an established risk factor for developing neurological and psychiatric disorders (Hammen, 2005; Chrousos, 2009). Moreover, increased stress responsiveness is implicated in the etiology of mood and anxiety disorders that differentially affect males and females (Goel and Bale, 2009; Solomon and Herman, 2009). Heightened stress sensitivity in women has been proposed as a key underlying factor in the development of depression (Becker et al., 2007). In accordance, marked sex dimorphisms in physiological and behavioral responses to stress are seen in both humans and animal models (Kudielka and Kirschbaum, 2005; Goel and Bale, 2009; Bangasser et al., 2010). For example, female rats have greater basal concentrations of corticosterone (CORT) and more persistent CORT responses to stress (Kitay, 1961; Bangasser and Valentino, 2014), and clinical studies indicate enhanced stress sensitivity and susceptibility to affective dysfunction in females (Kessler, 2003; Kudielka and Kirschbaum, 2005; Parker and Brotchie, 2010). These differences are thought to be mediated by sexual dimorphisms in the hypothalamic-pituitary-adrenal axis response to stress (Kudielka and Kirschbaum, 2005; Bangasser et al., 2010) and their possible interaction with the DA system (Dalla et al., 2008; Gillies et al., 2014). However, the effects of stress-related depression vulnerability on VTA DA neurons in females have not been examined.
In this study, we assessed VTA DA neuron activity in male and female rats at baseline, post- chronic mild stress (CMS), a widely used and well-validated animal model of stress-induced neurobehavioral adaptations relevant to depression (Willner, 2005; Hill et al., 2012), and post-forced swim test (FST), which, despite its use as an antidepressant evaluation tool, in itself constitutes an acute uncontrollable stressor, in control (CON) and CMS-exposed rats. In male rats, CMS increases forced swim test (FST) immobility duration and reduces the number of VTA DA neurons that are spontaneously active (i.e., population activity) without altering other parameters (i.e., firing rate, bursting) (Chang and Grace, 2014). Given these data and converging clinical and preclinical evidence suggesting that sex-dependent diversity in VTA function may contribute to differential rates of prevalence across many psychiatric disorders involving stress susceptibility and characterized by DA system malfunction (Gillies et al., 2014), we hypothesized that female rats would be more sensitive to stress-induced alterations in immobility behavior and VTA DA neuron activity.
Ketamine, a fast-acting antidepressant (Zarate et al., 2006), increases the number of escapes and restores normal VTA population activity in helpless rats (Belujon and Grace, 2014) and prevents the DA system downregulation observed following acute amphetamine withdrawal (Belujon et al., 2016). Moreover, an acute 10-mg/kg dose of ketamine has long-lasting antidepressant-like behavioral effects in rodents of both sexes (Carrier and Kabbaj, 2013; Franceschelli et al., 2015). For these reasons, we were interested in determining whether acute ketamine administration would have short-term effects on FST behavior and whether there were sustained effects on VTA activity in male and female rats. Consistent with an antidepressant-like profile, we hypothesized that ketamine would decrease FST immobility and restore VTA population activity, although these effects may differ in males and females.
Methods
Animals
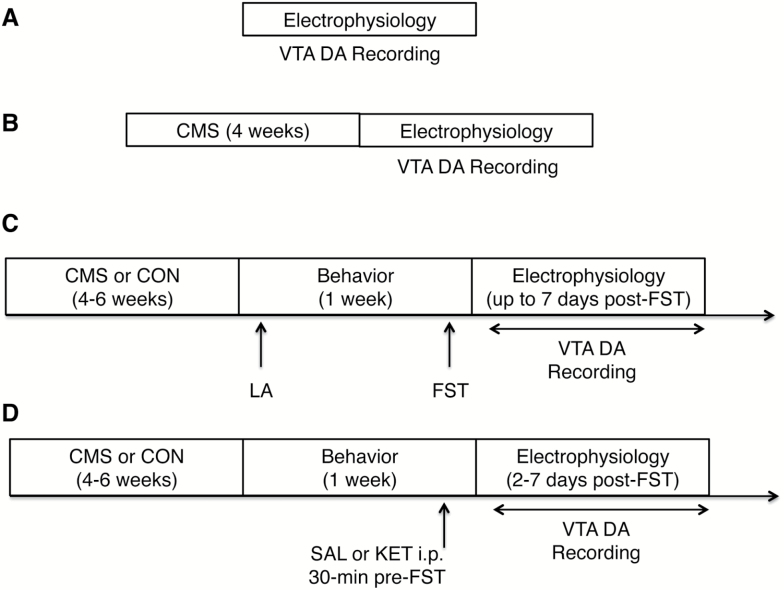
Adult male and female Sprague-Dawley rats (300-350 g and 220-270 g, respectively, at the beginning of the experiment) were used throughout this study. Animals (Envigo) were housed in pairs upon arrival for at least 5 days in a temperature- and humidity-controlled facility on a 12-hour-light/-dark cycle (lights on at 7:00 am) with food and water available ad libitum. All experiments were performed in accordance with the guidelines outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Experimental timeline for the use of animals is depicted in Figure 1. Vaginal smears, which would be required for long-term assessments of estrous cycle, were not collected during the CMS procedure or prior to behavioral testing so that the amount of handling was similar in both sexes. Thus, the aim of the present study was to investigate VTA activity function at baseline as well as the impact of CMS exposure in male and female rats, not the differences due to stage of the estrous cycle. Although the estrous cycle can impact behavior and neural function, a recent meta-analysis of 311 studies showed that randomly cycling female rats were not more variable than males across a range of behavioral and neurobiological processes, even when they were used without regard to estrous cycle stage (Becker et al., 2016).
Figure 1.
Experimental timeline. (A) In vivo extracellular ventral tegmental area (VTA) dopamine (DA) recordings were performed in both sexes under baseline (i.e., unstressed and experimentally naïve) conditions. (B) VTA DA recordings were conducted in a separate cohort of animals of both sexes following 4 weeks of chronic mild stress (CMS) exposure. (C) Male and female rats were exposed to 4 to 6 weeks of CMS or standard (CON) housing conditions prior to being tested for spontaneous locomotor activity and in the forced swim test (FST), which constitutes an acute uncontrollable stressor. VTA DA neuron recordings were conducted within 7 days post-FST. (D) A separate cohort of rats underwent 4 to 6 weeks of CMS or CON and received an i.p. injection of ketamine (KET, 10 mg/kg) or vehicle (SAL, 1 mg/kg) 30 minutes prior to the FST. VTA DA neuron recordings were conducted 2 to 7 days post-FST to identify long-lasting effects of KET on the VTA DA system. All VTA recordings were conducted using an acute prep in chloral hydrate anesthetized rats. Each experiment (A, B, C, D) represents separate cohorts. LA, locomotor activity.
CMS Procedure
The CMS procedure was adapted from previously published work (Chang and Grace, 2014) and consisted of a 4- to 6-week regimen in which rats were single-housed and randomly presented with 3to 4 mild stressors per week. Stressors included: food deprivation, water deprivation followed by 1-hour empty bottle presentation, light cycle reversal or disruption, cage tilts (45°), overnight stroboscopic lighting (ADJ 58I LED II), damp bedding (200-300 mL of lukewarm water in cage), foreign intruder, white noise (88 dB; continuous), and predator odor exposure (i.e., 20 μL undiluted 2,4,5-trimethylthiazoline for 1 hour). Age- and body weight-matched controls were housed in pairs over the equivalent period.
Behavioral Assays
Locomotor Activity
Animals were placed into an open-field arena (Coulbourn Instruments), and spontaneous locomotor activity was monitored for 10 minutes by beam breaks with TruScan software and indexed as the total distance travelled (cm) (Chang and Grace, 2014).
FST
The FST is a behavioral measure in rodents that has been widely used to assess the antidepressant-like effects of drugs (Porsolt et al., 1977; Cryan et al., 2002). Notably, this measure has been recently proposed to reflect a switch from active to passive coping in the face of an acute stressor rather than a depression-like phenotype (Molendijk and de Kloet, 2015). The FST took place in a clear Plexiglas cylinder (50 cm high, 20-cm diameter) filled with water (25°C) up to 38 to 40 cm. Animals received a 15-minute preexposure (day 1: habituation) on the day before the test. On test day (day 2), each rat was placed inside the cylinder for 5 minutes and immobility behavior, defined as making only minor necessary movements to maintain head above water (Porsolt et al., 1977), as well as the latency to the first (≥5 seconds) immobility bout was measured. Water was changed between animals on each of the days. Rats were removed and dried off before being placed back in their home cage.
Drug Administration
Ketamine (10 mg/kg, i.p.) or sterile saline (1 mL/kg, i.p.) was injected 30 minutes before the FST at a volume of 1 mL/kg (Carrier and Kabbaj, 2013) in a separate cohort of CON/CMS animals. This dose was selected due to its use in most preclinical ketamine studies as well as its long-lasting (~1 week) antidepressant behavioral effects in rodents (Browne and Lucki, 2013).
In Vivo Electrophysiological Recordings of VTA DA Neurons
Single-unit extracellular recordings were performed using an acute preparation in chloral hydrate anesthetized rats (Figure 1). We have previously reported CMS-induced changes in VTA activity in male rats up to 7 days post-FST) (Chang and Grace, 2014). Given that one of our aims was to assess whether these findings extended to females, we used the same amount of time with the recording order counterbalanced among groups. Rats were anesthetized with 8% chloral hydrate (400 mg/kg, i.p. Sigma), mounted on a stereotaxic frame (Kopf), and their body temperature was maintained at 37° using a temperature-controlled heating pad (Fine Science Tools). The skull was exposed and a burr hole was made to access the VTA using the following target coordinates referenced from bregma: anteroposterior [AP] -5.3 to 5.7 mm (males), -5.1 to 5.5 mm (females), mediolateral [ML] +0.6 to 1.0 mm from bregma and dorsoventral [DV] -6.5 to 9.0 mm from dura). Female coordinates were adjusted to -5.1 to 5.5 [AP] as determined through pilot work. Electrophysiological recordings were conducted using single-barrel glass electrodes filled with 2% Chicago Sky Blue (Sigma Aldrich) dissolved in 2 M saline, which were lowered into the VTA in a predetermined grid pattern of 6 to 9 tracks separated by 0.2 mm and spanning the anteroposterior (A/P) and mediolateral (M/L) extent (Supplementary Figure 1) (Grace and Bunney, 1983) using a manual hydraulic Microdrive (Kopf). This procedure was developed to sample dopamine neurons with a variety of different projection targets (Ikemoto, 2007) and has been published by our group in multiple prior studies (Valenti et al., 2012; Chang and Grace, 2014; Belujon et al., 2016; Moreines et al., 2017). Animals were required to have a minimum of 6 tracks within 0.4 mm of target coordinates to be included in the study. All recorded DA neurons were located -5.0 to -6.12 mm from bregma. Spontaneous neural activity was monitored in each track with open filter settings (50-16 kHz bandpass), and DA neurons were identified using well-established criteria including location, slow, irregular firing pattern, and long duration, variable shape biphasic action potential waveform (>2.2 milliseconds) and half-width (>1.1 milliseconds) (Grace and Bunney, 1983; Ungless and Grace, 2012). These criteria have also been used for DA neuron identification in females (Perez et al., 2014). Only identified DA neurons were recorded for 3 minutes (1.5-minute minimum) when signal-to-noise ratio exceeded 3:1.Three parameters of activity were measured: (1) population activity, or number of spontaneously active DA neurons per track; (2) basal firing rate; and (3) percent burst firing, or the proportion of action potentials occurring in bursts, with burst onset defined as 2 spikes with ≤80-millisecond interspike interval and termination by >160-millisecond interspike interval (Grace and Bunney, 1984). Electrode placement was verified via electrophoretic injection of Chicago Sky Blue dye at the recording site. Rats were killed with a lethal dose of chloral hydrate (additional 400 mg/kg, i.p.) and brains were removed following decapitation. The tissue was fixed in 8% paraformaldehyde for at least 48 hours and transferred to a 25% sucrose solution for cryoprotection. Once saturated, brains were frozen and sliced coronally at 60 µm thick using a cryostat (Leica Frigocut 2800) and mounted onto gelatin-chromalum-coated slides. Tissue was stained with a combination of neutral red and cresyl violet. Only rats with verified placement were included in the data analysis.
Analysis
Single-unit neuron data was analyzed with Powerlab Lab Chart (AD Instruments) to identify spike time courses and exported to Neuroexplorer (NEX Technologies, NexTech Systems) software to calculate firing rate and burst firing. Electrophysiological data were analyzed using t tests when the data was normally distributed and the Mann-Whitney U-test when data deviated from the normal distribution for pairwise comparisons or 2-way ANOVA. Behavioral results were analyzed by 2-way ANOVA. Posthoc comparisons were done using Tukey’s HSD for ANOVAs indicating a significant (P < .05) interaction and were considered significant when P < .05. All statistics were calculated with GraphPad Prism (Systat Software).
Results
VTA DA Neuron Activity Is Comparable in Male and Female Rats at Baseline
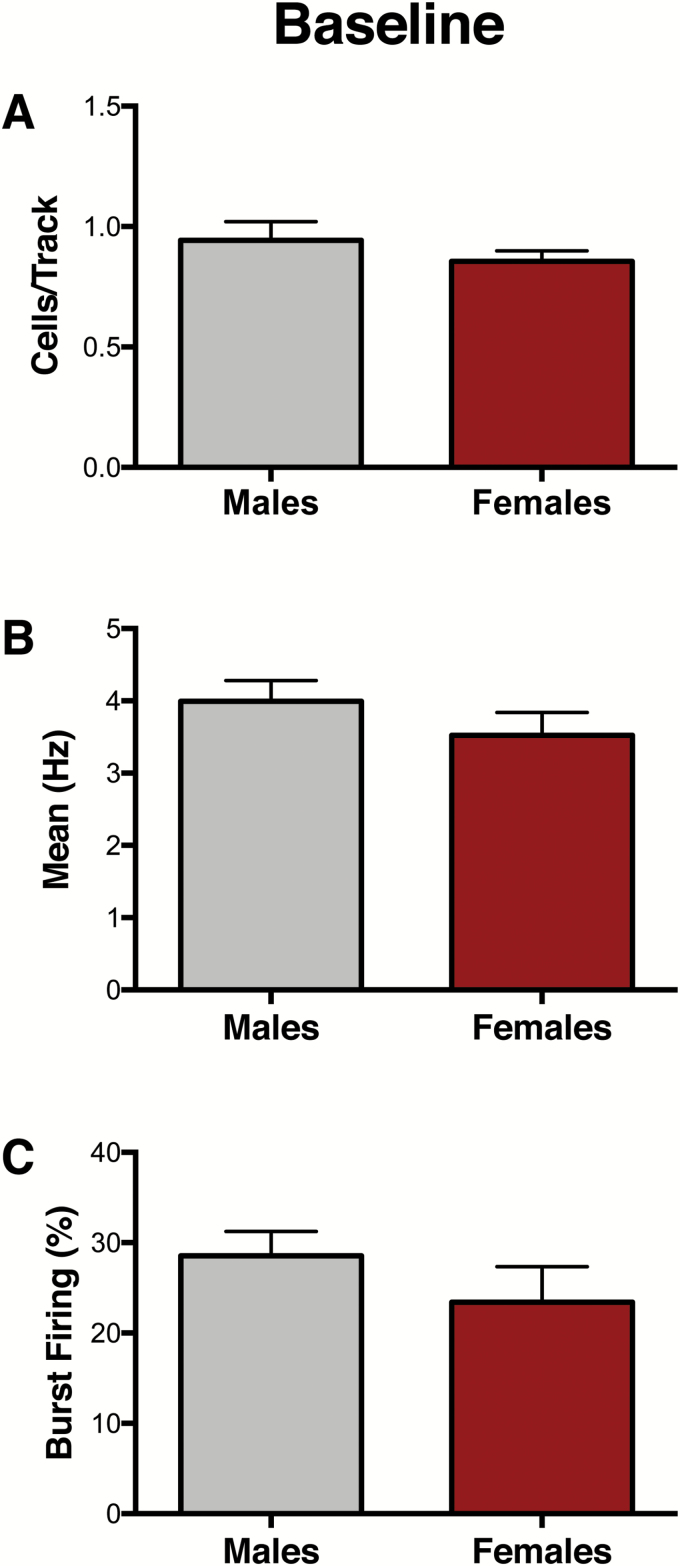
A cohort of experimentally naïve animals was used to conduct VTA recordings at baseline (Figure 1A). No differences between male (n=7) and female (n=7) rats were found for population activity, as indicated by the number of spontaneously active DA cells per track (CPT) (males: 0.94 ± 0.07 CPT, females: 0.86 ± 0.04 CPT; t12= 0.97, P=.35) (Figure 2A), firing rate (males: 3.99 ± 0.29 Hz, females: 3.52 ± 0.32 Hz; U=790, P=.42) (Figure 2B), or percentage of spikes firing in burst (%SIB) (males: 28.5 ± 2.7 %SIB, females: 23.4 ± 3.9 %SIB; U=828, P= .06) (Figure 2C). For representative VTA DA neuron activity in male and female rats, see supplementary Figure 2.
Figure 2.
Baseline ventral tegmental area (VTA) activity is similar in male and female rats. (A-C). At baseline, no significant differences between males (n=7) and females (n=7) were observed for (A) VTA population activity (P= .35), (B) firing rate (P= .42), or (C) percent of spikes firing in burst (P= .06). Error bars represent mean ± SEM. Gray bars represent males; red bars represent females.
CMS Exerts Greater Effects on VTA DA Neuron Activity in Female Rats
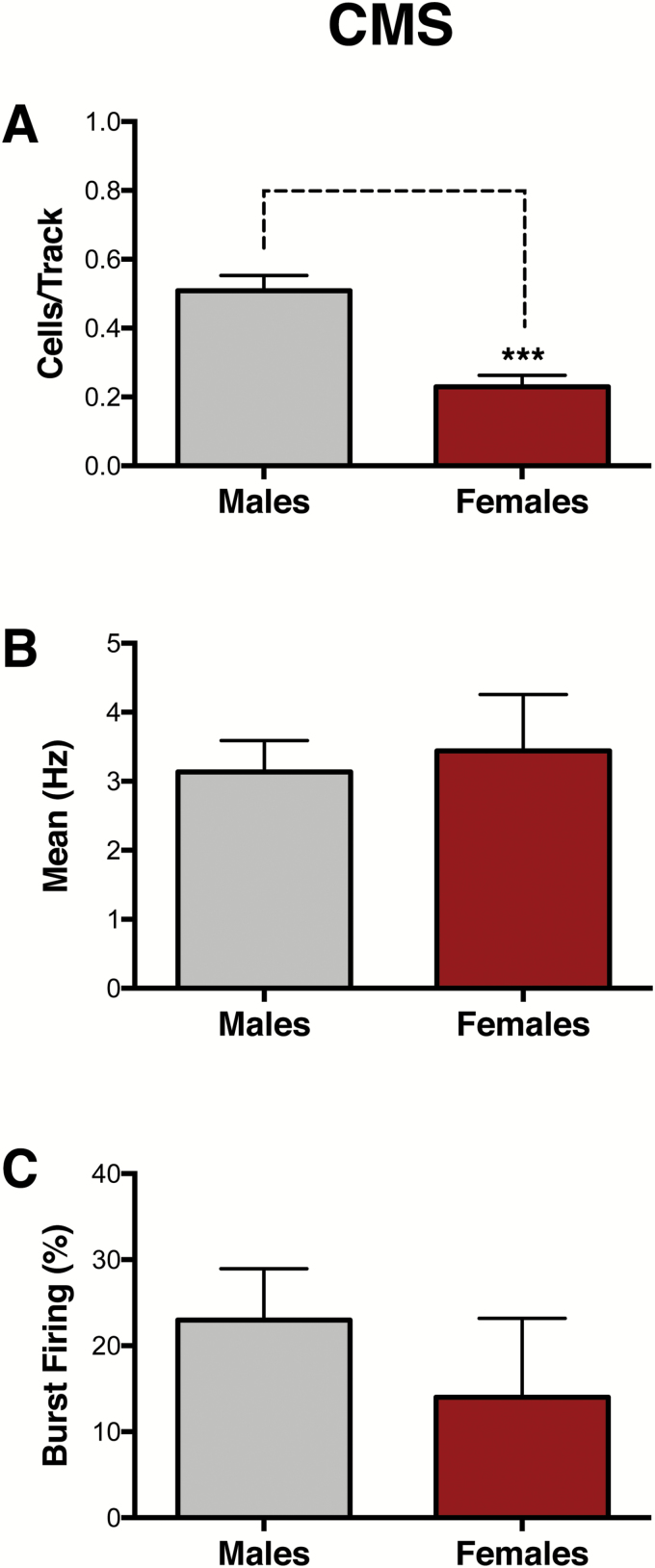
VTA recordings were conducted in a separate cohort of male and female rats that underwent 4 weeks of CMS to compare CMS effects between sexes (Figure 1B). Compared with male rats (n=6), which exhibit a reduction in the number of spontaneously active DA neurons in the VTA (Chang and Grace, 2014), females (n=6) exhibited a greater reduction in population activity of VTA DA neurons (males: 0.51 ± 0.04 CPT; females: 0.23 ± 0.04 CPT; t10= 5.02, P < .001) (Figure 3A) but no differences in average firing rate (males: 3.14 ± 0.45 Hz, females: 3.44 ± 0.81 Hz; t27= 0.35, P= .73) (Figure 3B) or average percentage of spikes fired in bursts (males: 23 ± 2.70 %SIB, females: 14 ± 9.17 %SIB; U=60, P= .17) (Figure 3C).
Figure 3.
Greater effects of chronic mild stress (CMS) on ventral tegmental area (VTA) population activity in females. A separate cohort of animals was exposed to 4 weeks of CMS to compare CMS effects between sexes. (A) Female rats exhibited reduced population activity compared with male rats (n = 6/group, P< .001). No differences were found in (B) firing rate (P= .73) or (C) bursting activity (P= .17). Error bars represent mean ± SEM. Gray bars represent males; red bars represent females. ***P< .001.
Acute and Chronic Stressors Exert Sex-Dependent Effects on Behavior and VTA DA Neuron Activity
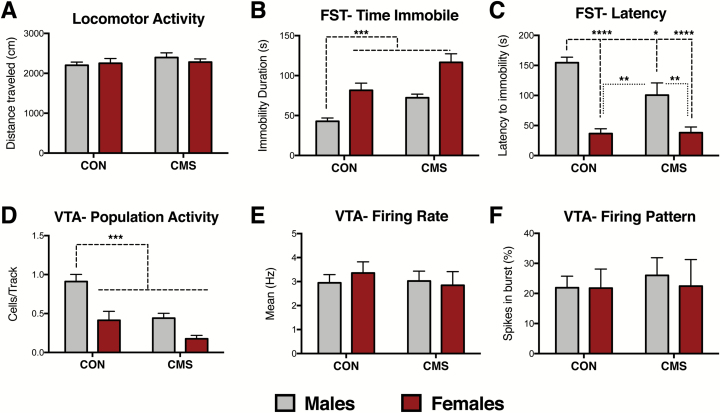
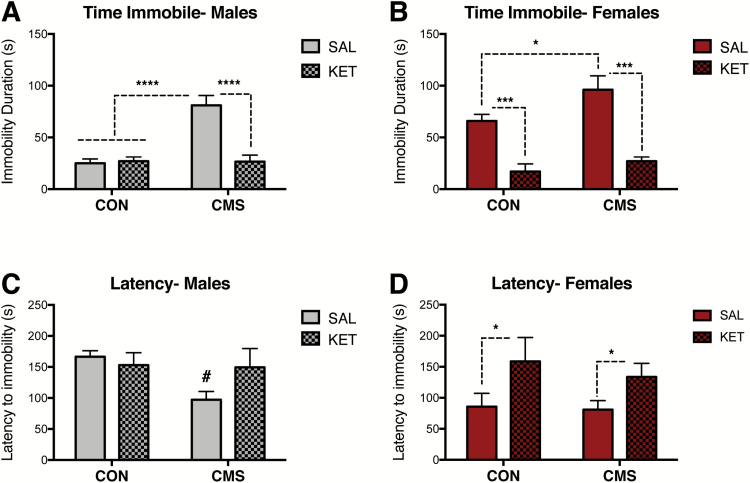
A separate cohort of rats underwent behavioral testing (locomotor activity, FST) after 4 to 6 weeks of CON or CMS conditions (Figure 1C). Two-way ANOVA indicated no effects of sex (F1,42= 0.10, P = .75) or CMS (F1,42= 1.22, P = .27) on locomotor activity, as indexed by distance traveled (CON groups n=12, CMS groups n=11) (Figure 4A). These same animals underwent the FST within the same week, which was performed one day following a 15-minute FST habituation exposure period (Porsolt et al., 1977). Two-way ANOVA revealed significant effects of both sex (F1, 42= 0.13, P < .0001) and CMS (F1, 42= 18.05, P < .001) on immobility duration (Figure 4B). While CON males exhibited lower immobility duration compared with CON females and CMS groups, CON females exhibited comparable immobility levels with CMS groups. Significant effects of sex (F1, 41= 51.12, P < .0001) and CMS (F1, 41= 4.35, P < .05) were also found for latency to immobility (Figure 4C). An interaction between sex and CMS was also observed (F1, 41= 4.82, P<.05) for latency to immobility. Posthoc analysis revealed that CON males exhibited longer latency to immobility compared with CON females and CMS groups; CMS females did not differ from CON females.
Figure 4.
Female rats exhibited greater behavioral and ventral tegmental area (VTA) dopamine (DA) neuron activity responses post-forced swim test (FST). (A) No effects of sex (P= .75) or chronic mild stress (CMS) (P= .27) were found for locomotor activity (CON groups: n = 12, CMS groups: n = 11). (B) Females exhibit higher immobility duration than males in the FST; CMS exposure increases immobility in the FST (CON groups: n = 12, CMS groups: n = 11; significant effects of both sex [P< .001] and CMS [P< .001]). (C) Females exhibit reduced latency to immobility compared with males in the FST; CMS reduces latency to immobility [(CON groups: n=12, CMS groups: n=11; significant effects of both sex (P< .0001) and CMS (P< .05) and an interaction between sex and CMS (P< .05)]. (D) Post-FST, females exhibited lower numbers of spontaneously active VTA DA neurons (i.e., cells per track) compared with males, consistent with a sex difference in response to swim stress. CMS reduced the number of spontaneously active DA neurons post-FST. CMS males and females exhibited reduced cells/track compared with CON males (significant effects of sex [P< .001] and CMS [P< .001] on spontaneous VTA DA neuron activity (CON groups: n = 6, CMS males: n = 7, CMS females: n = 5). Notably, these recordings were done in animals that underwent the FST. (E) No effects of sex (P= .82) or CMS (P= .67) were found for firing rate. (F) No effects of sex (P= .78) or CMS (P= .71) on percent of neurons firing in burst. Error bars represent mean ± SEM. Gray bars represent males; red bars represent females. *P< .05, **P< .01, ***P< .001, ****P< .0001.
VTA recordings were conducted within 7 days post-FST in the same animals used for behavior, as we have previously reported CMS-induced changes in VTA activity within this period (Chang and Grace, 2014). Post-FST exposure, effects of sex (F1, 20= 20.05, P < .001) and CMS (F1, 20= 17.34, P < .001) were found for VTA population activity (Figure 4D). Compared with CON males, CON females exhibited a dramatic reduction in the number of spontaneously active VTA DA neurons post-FST (CON males: n=6 animals, 0.91 ± 0.09 CPT; CON females: n=6 animals, 0.41 ± 0.11 CPT). CMS reduced VTA DA neuron population activity in males (n=7, 0.44 ± 0.06 CPT) and females (n=5, 0.18 ± 0.04 CPT). No effects of unaffected sex (F1, 86= 0.33, P=.82) or CMS (F1, 86= 0.18, P=.67) were found for firing rate (Figure 4E). No effects of sex (F1, 86= 0.08, P=.78) or CMS (F1, 86= 0.13, P=.71) were found in percentage of spikes fired in bursts (Figure 4F).
Ketamine Reduces Immobility Behavior and Exerts Long-Lasting Restorative Effects on VTA DA Neurons
A separate cohort of rats underwent 4 to 6 weeks of CMS or CON and received an i.p. injection of ketamine (KET, 10 mg/kg) or vehicle (SAL, 1 mg/kg) 30 minutes prior to the FST (Figure 1D). VTA DA neuron recordings were conducted 2 to 7 days post-FST in these animals to identify post-acute, long-lasting effects of KET on the VTA DA system. Two-way ANOVA revealed significant effects of CMS (F1, 29= 21.96, P<.0001) and drug (F1, 29= 19.71, P<.001), as well as an interaction between CMS and drug (F1, 29=22.81, P<.0001) on immobility behavior in male rats (CON-SAL: n=9, CON-KET: n=10, CMS groups: n=7/group) (Figure 5A). Posthoc analyses indicated that SAL-treated CMS males were significantly different from all other groups. In males, no effects of stress (F1, 29= 3.56, P=.07) or drug (F1, 29= 1.01, P=.32) on latency to immobility were found (CON-SAL: n=9, CON-KET: n=10, CMS groups: n=7/group) (Figure 5C). Effects of CMS (F1, 26= 5.16, P<.05) and drug (F1, 26= 44.34, P<.001) on immobility behavior were also found in female rats (CON-SAL: n=8, CON-KET: n=6, CMS groups: n=8/group) (Figure 5B). Ketamine administration following FST habituation but 30 minutes prior to FST testing reduced immobility in both CON and CMS females. A significant effect of drug (F1, 26= 6.92, P<.05) was found in females (CON-SAL: n=8, CON-KET: n=6, CMS groups: n=8/group) (Figure 5D).
Figure 5.
Antidepressant-like effects of acute ketamine administration on the forced swim test (FST). (A) Ketamine reduced immobility duration in male rats exposed to chronic mild stress (CMS) (CON-SAL: n=9, CON-KET: n=10, CMS groups: n=7/group; P< .0001). (B) Ketamine reduced immobility duration in CON and CMS female rats exposed to the FST (CON-SAL: n=8, CON-KET: n=6, CMS groups: n=8/group; P< .001). (C) Ketamine did not affect latency to immobility in male rats (CON-SAL: n=9, CON-KET: n=10, CMS groups: n=7/group; P= .32). (D) Ketamine increased the latency to immobility in CON and CMS female rats (CON-SAL: n=8, CON-KET: n=6, CMS groups: n=8/group; P< .05). Error bars represent mean ± SEM. Gray bars represent males; red bars represent females; checkered bars represent ketamine-treated groups. *P< .05, **P< .01, ***P< .001, ****P< .0001, # denotes trend (P= .06).
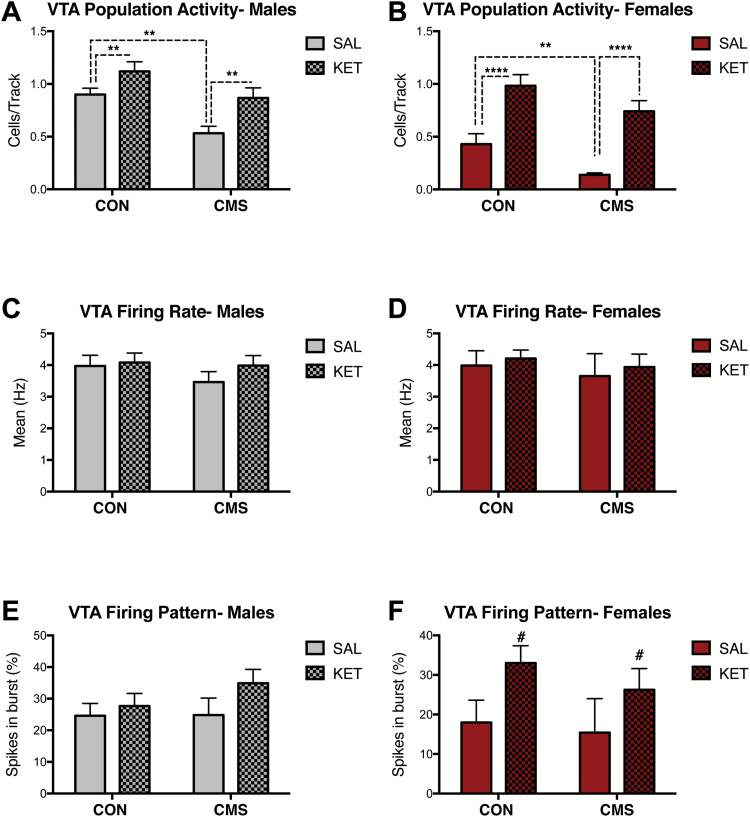
In males, significant effects of CMS (F1, 17= 13.63, P<.01) and drug (F1, 17= 10.87, P<.01) were found on population activity (SAL CON: n=4, 0.90 ± 0.06 CPT; SAL CMS: n=6, 0.53 ± 0.07 CPT; KET CON: n=5, 1.1 ± 0.09 CPT; KET CMS: n=6, 0.87 ± 0.97 CPT) (Figure 6A), but not firing rate (CMS: F1,131= 0.81, P=.37; drug: F1,131= 0.89, P=.35) (Figure 6C) or percentage of spikes fired in bursts (CMS: F1,129= 0.66, P=.42; drug: F1,129= 2.07, P=.15) 2 to 7 days post ketamine administration (Figure 6E). Significant effects of CMS (F1, 19= 9.13, P<.01) and drug (F1, 19= 42.56, P<.0001) on VTA population activity were also found for females (SAL CON: n=5, 0.43 ± 0.10 CPT; SAL CMS: n=6, 0.14 ± 0.02 CPT; KET CON: n=6, 0.98 ± 0.10 CPT; KET CMS: n=6, 0.74 ± 0.10 CPT) during this same period (Figure 6B). No effects of CMS (F1,88= 0.42, P=.52) or drug (F1,88= 0.29, P=.59) were found for firing rate in females (Figure 6D). No effects of CMS (F1,87= 0.46, P=.50) were found in females, although a trend for effects of drug on percentage of spikes in burst (F1,87= 3.56; P=.06) was observed (Figure 6F).
Figure 6.
Ketamine has long-term (i.e., 2-7 days post-administration) effects on ventral tegmental area (VTA) dopamine (DA) neurons in male and female rats. (A) Ketamine increased population activity in male rats exposed to chronic mild stress (CMS) (SAL CON: n=4, SAL-CMS: n=6, KET CON: n=5, KET-CMS n=6; P< .01). (B) Ketamine increased VTA population activity in females (SAL CON: n=5, SAL-CMS: n=6, KET CON: n=6, KET-CMS n=6; P< .0001). (C) Ketamine administration did not alter firing rates males (P=.35). (D) No effect of ketamine on firing rate in females (P=.59). (E) Ketamine administration did not change the percentage of burst firing in males (P=.15). (F) Ketamine administration did not change the percentage of burst firing in females (P=.06). Error bars represent mean ± SEM. Gray bars represent males; red bars represent females; checkered bars represent ketamine-treated groups. *P< .05, **P< .01, ***P< .001, ****P< .0001, # denotes trend (P= .06).
Discussion
We conducted the first in vivo electrophysiological study of VTA DA neurons in rats of both sexes at baseline, post-CMS, and post-FST. Although VTA DA system function was comparable between the sexes at baseline, we identified sex-dependent effects of stress in behavior and DA neuron activity. Consistent with prior reports, female rats exhibited greater FST immobility and reduced latency to immobility compared with males (Drossopoulou et al., 2004; Dalla et al., 2008; Kokras et al., 2015), which was also observed in CMS-exposed rats. Although immobility levels during the pre-test FST are similar in both sexes (Drossopoulou et al., 2004; Dalla et al., 2011), increased immobility duration in females emerges during the test swim session and is associated with reduced swimming, climbing, and head-swinging behaviors (Drossopoulou et al., 2004; Dalla et al., 2008; Kokras et al., 2015). In naturally cycling females, immobility levels are similar across the different stages of the estrous cycle, and immobility behavior, swimming, climbing are not correlated with hormonal serum levels or uterus weight, suggesting that the estrous cycle effect on forced swim behavior is small (Kokras et al., 2015). Furthermore, prior reports have also suggested no impact of estrous stage on FST in adult Sprague Dawley females (Liang et al., 2008; Craft et al., 2010; D’Aquila et al., 2010). The contribution of estrous cycle to the observed behavioral effects in CMS females remains unclear, given that CMS desynchronizes estrous cycling across multiple strains, including Sprague Dawley (Dalla et al., 2005; Grippo et al., 2005; Baker et al., 2006; Lu et al., 2015). These data demonstrate that a single forced swim exposure in female rats, unlike males, is sufficient to induce a depressive-like state measured behaviorally and electrophysiologically.
Post-FST, females display increased CORT levels compared with males (Drossopoulou et al., 2004), thereby suggesting a link between heightened stress reactivity (i.e., CORT secretion) and increased FST immobility in female rats. This is consistent with our behavioral results demonstrating increased FST immobility following CMS exposure. CMS dysregulates the HPA-axis and elevates CORT levels in both sexes post-FST (Dalla et al., 2005; Dalla et al., 2011). Importantly, glucocorticoids released in response to the FST are necessary for the incorporation of a behavioral response and modulate immobility behavior (Veldhuis et al., 1985; Mitchell and Meaney, 1991). Furthermore, a stressful history is known to sensitize animals to the acute forced swim stressor itself (Gray et al., 2014), and the enhanced immobility response following chronic stress is thought to be a consequence of increased stress responsiveness (Molendijk and de Kloet, 2015). Female rodents display elevated CORT levels at baseline (Kitay, 1961; Drossopoulou et al., 2004), in response to single housing (Brown and Grunberg, 1995)—a component of our CMS procedure—as well higher stress-induced CORT levels (Bangasser and Valentino, 2014), which may contribute to the exacerbation of stress effects in females. Similarly, depressed women typically have higher cortisol levels than depressed men, and this sex difference is particularly pronounced following stressful or negative events (Bangasser and Valentino, 2014).
At baseline, male and female rats exhibited comparable VTA DA activity across the 3 parameters evaluated: population activity, firing rate, and firing pattern. CMS caused a greater reduction in VTA population activity (i.e., the number of spontaneously active DA neurons) in female rats compared with males. Acute stress (i.e., FST exposure) reduced population activity by approximately 40% to 50% in CON female rats, an effect that was absent in post-FST CON males. Surprisingly, VTA population activity in CON females post-FST was comparable with those seen in CMS groups post-FST exposure. The lack of a further reduction of VTA DA neurons in CMS females post-FST may be because females already show a markedly greater reduction in VTA population activity post-CMS. Taken together, these findings suggest a sex-dependent effect of swim stress and CMS on VTA population activity in which females are more susceptible to stress-induced DA downregulation. Whether these changes are specific to the type/saliency of stressors used here (FST, CMS) remains to be determined. Since different stressors may have opposite effects (i.e., increase or decrease) on VTA DA neuronal activity depending on a number of factors, including stressor severity (Valenti et al., 2012; Holly and Miczek, 2016), future studies looking at the effects of different type/saliency of stressors on VTA DA activity in both sexes are needed. Given recent evidence demonstrating that naïve female rats exhibit comparable DA neuron population activity across the estrous cycle (Perez et al., 2014), and that females, even when used without regard to the estrous cycle stage, are not more variable than males (Bale and Epperson, 2016; Becker et al., 2016), we simply accepted circulating gonadal hormones in both sexes as part of the complex physiological background of each animal (Shansky and Woolley, 2016).
VTA DA neurons show activity states that have important implications for DA system function: tonic (i.e., population) activity and bursting (i.e., phasic) activity in response behaviorally salient stimuli (Grace, 2016). However, a DA neuron must already be spontaneously active to transition into burst firing; the number of spontaneously active DA neurons (i.e., tonic or population activity) being controlled by a potent GABAergic input from the ventral pallidum (VP) (Floresco et al., 2003). Thus, population activity sets the baseline tone of DA system responsivity and modulates the amplitude of the phasic response (Lodge and Grace, 2006; Grace, 2016). Our current findings regarding stress effects on the DA system are in accordance with prior work by our group showing that learned helplessness and CMS dramatically decrease the number of active VTA DA neurons in male rats (Belujon and Grace, 2014; Chang and Grace, 2014), which would diminish the amplitude of the phasic burst firing response to salient, reward-related stimuli and is thought to drive depressive symptoms (Lodge and Grace, 2006; Belujon and Grace, 2015). Indeed, inhibition of VTA DA neurons can acutely induce multiple depression-like behaviors, and activation of these neurons can rescue CMS-induced depression-like phenotypes (Tye et al., 2013). Importantly, the CMS-induced decrease in DA neuron population activity is reversed by pharmacological inactivation of the basolateral amygdala (BLA) or the VP in male rats (Chang and Grace, 2014). Pharmacological activation of the amygdala in nonstressed rats mimics the effects of CMS on VTA population activity; blocking glutamate inputs into the VP prevents this BLA activation-induced decrease in DA activity. Collectively, these findings highlight a BLA-VP-VTA circuit that mediates CMS-induced inhibition of DA neuron activity.
Finally, a 10-mg/kg ketamine dose given prior to the FST prevents the increased FST immobility and causes a sustained increase in VTA population activity in males and females. Using optogenetic techniques, Tye et al. 2013 have observed a robust increase in escape-related behavior (i.e., kick frequency) during the FST in CMS rats following activation of VTA DA neurons, which was not time locked to the light pulses, suggesting a role for dopaminergic tone (i.e., population activity), rather than bursting activity, in this behavior. This is consistent with the observed behavioral (i.e., reduced immobility) and electrophysiological (i.e., increased population activity) effects of ketamine, although our recent work suggests that DA neuron activity and FST immobility are correlated, but not dependent on each other (Belujon et al., 2016). Although the effects of ketamine on locomotor activity have been mixed, likely due to differences in age and strain, multiple studies have reported no effect of this dose of ketamine on locomotor activity in an open field 20 to 30 minutes post-administration in adult rats of a variety of strains (Browne and Lucki, 2013; Belujon and Grace, 2014), including Sprague Dawley (Wilson et al., 2007; Engin et al., 2009), regardless of sex (Wilson et al., 2007; Browne and Lucki, 2013). In females, ketamine also increased latency to immobility. Female rats and mice are more sensitive to the antidepressant-like behavioral effects of ketamine and respond to lower (i.e., 2.5-3 mg/kg) doses of ketamine than males (Carrier and Kabbaj, 2013; Franceschelli et al., 2015). Indeed, a recent study found that ketamine’s antidepressant actions are mediated by a metabolite, hydroxynorketamine, which is several-fold higher in the brains of female than male rodents after receiving the same dose (Zanos et al., 2016).
Previous studies done by our group have shown no differences between acute (i.e., immediate) and long-lasting effects of ketamine in rodent models of depression using our measure (i.e., cells/track) (Belujon and Grace, 2014; Belujon et al., 2016). For example, ketamine increases population activity in helpless animals 20 minutes, 2 hours, and 24 hours post-injection (Belujon and Grace, 2014). Our results in CMS animals recorded between 2 and 7 days post-ketamine are consistent with this extended, rather than acute action of this drug. The time course of the protracted effects of ketamine on VTA DA neurons resembles the time course for clinical effects, as acute ketamine treatment produces rapid antidepressant effects that last up to 7 days in humans (Zarate et al., 2006), and is consistent with the time course of antidepressant-like behavioral effects across multiple rodent models of depression (Browne and Lucki, 2013; Franceschelli et al., 2015), suggesting that some of these effects may be mediated via long-lasting actions on the DA system.
In sum, although VTA DA system function was comparable between the sexes at baseline, CMS exposure had a greater effect on VTA population activity in females. Acute stress (i.e., FST exposure) decreased VTA population activity in females but not in males, whereas CMS increased FST immobility and dramatically dampened the number of spontaneously active DA cells in the VTA in both sexes; ketamine prevented these effects. Our findings provide insight into sex differences in stress-induced adaptation in DA system function, which may contribute to sex-specific treatment of psychiatric disorders involving stress susceptibility, DA system malfunction and characterized by sex differences in their prevalence or nature.
Funding
Research reported in this publication was supported by the National Institute of Mental Health of the National Institute of Health under award numbers T32-MH016804 and F32-MH110128 to M.R.C. and R01-MH101180 to A.A.G.
Statement of Interest
Dr. Rincón-Cortés reports no potential conflicts of interest. Dr. Grace receives consulting fees from Johnson & Johnson, Lundbeck, Pfizer, GSK, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, Asubio, Alkermes, and Abbott and receives research funding from Lundbeck, Autofony, and Johnson & Johnson.
Supplementary Material
Acknowledgments
We thank Nicole MacMurdo and Christy Smolak for technical assistance with histology.
References
- Baker SL, Kentner AC, Konkle AT, Santa-Maria Barbagallo L, Bielajew C (2006) Behavioral and physiological effects of chronic mild stress in female rats. Physiol Behav 87:314–322. [DOI] [PubMed] [Google Scholar]
- Bale TL, Epperson CN (2016) Sex as a biological variable: who, what, when, why and how. Neuropsychopharmacology 42:386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ (2010) Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:877, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ (2014) Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 35:303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Monteggia LM, Perrot-Sinal TS, Romeo RD, Taylor JR, Yehuda R, Bale TL (2007) Stress and disease: is being female a predisposing factor? J Neurosci 27:11851–11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Prendergast BJ, Liang JW (2016) Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I (2011) Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA (2014) Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA (2015) Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc Biol Sci 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Jakobowski NL, Dollish HK, Grace AA (2016) Withdrawal from acute amphetamine induces an amygdala-driven attenuation of dopamine neuron activity: reversal by ketamine. Neuropsychopharmacology 41:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KJ, Grunberg NE (1995) Effects of housing on male and female rats: crowding stresses male but calm females. Physiol Behav 58:1085–1089. [DOI] [PubMed] [Google Scholar]
- Browne CA, Lucki I (2013) Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 4:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M (2013) Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70:27–34. [DOI] [PubMed] [Google Scholar]
- Chang CH, Grace AA (2014) Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry 76:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, et al. (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493:532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. (2009) Stress and disorders of the stress system. Nat Rev Endocrinol 5:374–381. [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS (2014) Policy: NIH to balance sex in cell and animal studies. Nature 509:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Kostick ML, Rogers JA, White CL, Tsutsui KT (2010) Forced swim test behavior in postpartum rats. Pharmacol Biochem Behav 96:402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23:238–245. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z (2005) Chronic mild stress impact: are females more vulnerable? Neuroscience 135:703–714. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, Daskas S, Papadopoulou-Daifoti Z (2008) Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav 93:595–605. [DOI] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z (2011) Sex differences in response to stress and expression of depressive-like behaviours in the rat. Curr Top Behav Neurosci 8:97–118. [DOI] [PubMed] [Google Scholar]
- D’Aquila PS, Canu S, Sardella M, Spanu C, Serra G, Franconi F (2010) Dopamine is involved in the antidepressant-like effect of allopregnanolone in the forced swimming test in female rats. Behav Pharmacol 21:21–28. [DOI] [PubMed] [Google Scholar]
- Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, Papadopoulou-Daifoti Z (2004) Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience 126:849–857. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D, Dickson CT (2009) Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience 161:359–369. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6:968–973. [DOI] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM (2015) Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neuroscience 290:49–60. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Virdee K, McArthur S, Dalley JW (2014) Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: a molecular, cellular and behavioral analysis. Neuroscience 282C:69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Bale TL (2009) Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol 21:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. (2016) Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 17:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS (1983) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience 10:301–315. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS (1984) The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4:2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JD, Rubin TG, Hunter RG, McEwen BS (2014) Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry 19:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD (2005) Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl) 179:769–780. [DOI] [PubMed] [Google Scholar]
- Hammen C. (2005) Stress and depression. Annu Rev Clin Psychol 1:293–319. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J (2012) Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev 36:2085–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Miczek KA (2016) Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology (Berl) 233:163–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. (2001) Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord 62:77–91. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56:27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. (1993) Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev 18:75–113. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA (1999) Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841. [DOI] [PubMed] [Google Scholar]
- Kessler RC. (2003) Epidemiology of women and depression. J Affect Disord 74:5–13. [DOI] [PubMed] [Google Scholar]
- Kitay JI. (1961) Sex differences in adrenal cortical secretion in the rat. Endocrinology 68:818–824. [DOI] [PubMed] [Google Scholar]
- Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C (2015) Forced swim test: What about females? Neuropharmacology 99:408–421. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C (2005) Sex differences in HPA axis responses to stress: a review. Biol Psychol 69:113–132. [DOI] [PubMed] [Google Scholar]
- Liang S, Byers DM, Irwin LN (2008) Sex and diet affect the behavioral response of rats to chronic mild stressors. Physiol Behav 93:27–36. [DOI] [PubMed] [Google Scholar]
- Lloyd C. (1980) Life events and depressive disorder reviewed. II. Events as precipitating factors. Arch Gen Psychiatry 37:541–548. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA (2006) The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology 31:1356–1361. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu XY, Zhu QB, Li J, Shi LG, Wu JL, Zhang QJ, Huang ML, Bao AM (2015) Sex differences in the stress response in SD rats. Behav Brain Res 284:231–237. [DOI] [PubMed] [Google Scholar]
- Marinelli M, McCutcheon JE (2014) Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 282C:176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C. (2009) Is depression associated with dysfunction of the central reward system? Biochem Soc Trans 37:313–317. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Meaney MJ (1991) Effects of corticosterone on response consolidation and retrieval in the forced swim test. Behav Neurosci 105:798–803. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, de Kloet ER (2015) Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 62:389–391. [DOI] [PubMed] [Google Scholar]
- Moreines JL, Owrutsky ZL, Grace AA (2017) Involvement of infralimbic prefrontal cortex but not lateral habenula in dopamine attenuation after chronic mild stress. Neuropsychopharmacology 42:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159. [DOI] [PubMed] [Google Scholar]
- Parker G, Brotchie H (2010) Gender differences in depression. Int Rev Psychiatry 22:429–436. [DOI] [PubMed] [Google Scholar]
- Perez SM, Chen L, Lodge DJ (2014) Alterations in dopamine system function across the estrous cycle of the MAM rodent model of schizophrenia. Psychoneuroendocrinology 47:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732. [DOI] [PubMed] [Google Scholar]
- Seney ML, Sibille E (2014) Sex differences in mood disorders: perspectives from humans and rodent models. Biol Sex Differ 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Woolley CS (2016) Considering sex as a biological variable will be valuable for neuroscience research. J Neurosci 36:11817–11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Herman JP (2009) Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav 97:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K (2013) Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA (2012) Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Gill KM, Grace AA (2012) Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. Eur J Neurosci 35:1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis HD, De Korte CC, De Kloet ER (1985) Glucocorticoids facilitate the retention of acquired immobility during forced swimming. Eur J Pharmacol 115:211–217. [DOI] [PubMed] [Google Scholar]
- Willner P. (2005) Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110. [DOI] [PubMed] [Google Scholar]
- Wilson C, Kercher M, Quinn B, Murphy A, Fiegel C, McLaurin A (2007) Effects of age and sex on ketamine-induced hyperactivity in rats. Physiol Behav 91:202–207. [DOI] [PubMed] [Google Scholar]
- Yadid G, Friedman A (2008) Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res 172:265–286. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.