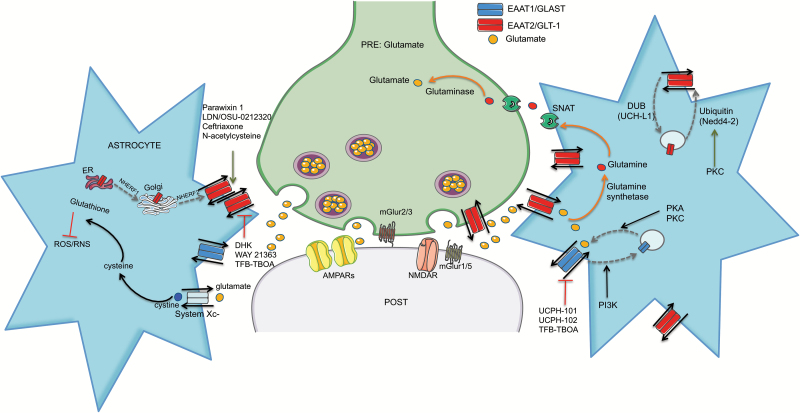
Figure 2.
Glial glutamate transporters. Excitatory amino acid transporter EAAT1/GLAST (blue) is located exclusively on astrocytes and other glial cells. EAAT2/GLT-1 (red) is likewise predominately restricted to astrocytes. Orange arrows depict the glutamate-glutamine cycle associated with glutamate uptake: glutamine synthetase converts up taken glutamate to glutamine, glutamine is transported back to the glutamatergic neuron through sodium coupled amino acid transporters (SNATs), and glutamine is converted back to glutamate by glutaminase. The gray dashed arrows depict regulation of EAAT trafficking. Endosomal trafficking of EAAT1 depends on sodium-hydrogen exchanger regulatory factor 1 and 2 (NHERF1 and 2). Rapid cell surface expression of EAATs is also prominently modulated by kinase activity. Protein Kinase A (PKA) and Protein Kinase C (PKC) inhibitors decrease EAAT1 surface expression, while Phosphoinositide 3-Kinase (PI3K) inhibitors promote surface expression. EAAT2 trafficking is regulated both constitutively and inducibly by ubiquitination/deubiquitination cycles. PKC-dependent activation of Neural precursor cell-expressed developmentally downregulated gene 4-2 (Nedd4-2) ubiquitin ligase targets EAAT2 for proteasomal degradation. Specific pharmacological activators (green arrows) and inhibitors (red bar-line) of each transporter are shown. UCPH-101 and UCPH-102 are the first selective EAAT1 inhibitors. TFB-TBOA ((3S)-3-[[3-[[4-(Trifluoromethyl)benzoyl]amino]phenyl]methoxy]-L-aspartic acid) is more selective for EAAT1 and EAAT2 but has low efficacy at EAAT3 as well. Dihydrokainate (DHK) blocks EAAT2 function. WAY-213613 is a competitive inhibitor with higher selectivity and potency for EAAT2 over EAAT1 and EAAT3. Parawixin 1, purified from the venom of spider Parawixia bistriata, selectively increases EAAT2 activity. Pyradizine analogs, including LDN/OSU-0212320 increases EAAT2 function through transactivation. Ceftriaxone and other beta-lactam antibiotics increase EAAT2 expression and function. The antioxidant pro-drug N-acetylcysteine (NAC) increases EAAT2 function but may interact with the glutamate transport system at multiple levels.