Table 1.
Identification of a catalytic system for the carbocyclization of unactivated alkyl bromides.
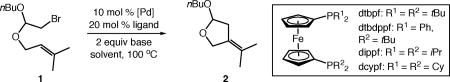
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | catalyst | ligand | base | solvent | yield (%)a |
| 1 | [Pd(allyl)Cl]2 | IMes | iPr2NEt | PhMe | <2 |
| 2b | Pd(PPh3)4 | – | PMP | PhtBu | <2 |
| 3 | Pd(OAc)2 | dtbdppf | iPr2NEt | PhMe | 5 |
| 4 | Pd(OAc)2 | dcypf | iPr2NEt | PhMe | <2 |
| 5 | Pd(OAc)2 | dippf | iPr2NEt | PhMe | <2 |
| 6 | Pd(OAc)2 | dtbpf | iPr2NEt | PhMe | 30(3) |
| 7 | [Pd(allyl)Cl]2 | dtbpf | iPr2NEt | PhMe | 65(12) |
| 8 | [Pd(allyl)Cl]2 | dtbpf | Et3N | PhMe | 73(5) |
| 9 | [Pd(allyl)Cl]2 | dtbpf | Et3N | PhCF3 | 85(5) |
| 10 | Pd(dtbpf)Cl2 | – | Et3N | PhCF3 | 14(2) |
Yield determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as internal standard. Yield in parenthesis is that of a minor regioisomer, see Supporting Information for details.
Reaction temperature 130 °C.
