Table 3.
Catalytic carbocyclization of diverse unactivated alkyl bromides.
| entry | substrate | product | yield (%)a |
|---|---|---|---|
| 1 |
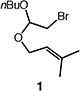
|
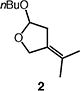
|
86b |
| 2 |
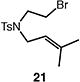
|
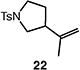
|
81b |
| 3 |
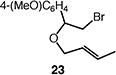
|
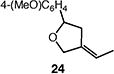
|
71 (1:1 E:Z) |
| 4 |
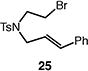
|
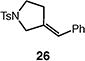
|
65 (1:1 E:Z) |
| 5c |
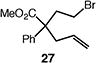
|
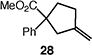
|
65 |
| 6d |
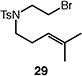
|
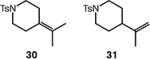
|
92 (30:31 = 1.9:1) |
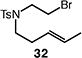
| 7d |
|
|
70 (33:34 = 3.3:1) |
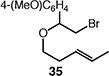
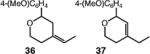
| 8d |
|
|
40 (36:37 = 7.0:1) |
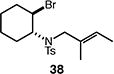
| 9 |
|
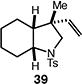
|
63 (>95:5 dr) |
| 10 |
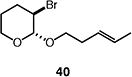
|
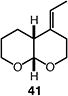
|
75 (>95:5 dr) (1.5:1 E:Z) |
Reactions were performed with [substrate]0 = 0.25 M in PhCF3 at 100 °C with 5 mol % [Pd(allyl)Cl]2, 20 mol % dtbpf, and 2 equiv Et3N as base.
Isolated yield.
Isolated with minor regioisomers, see Supporting Information for details.
DBU used as base instead of Et3N.
Reactions were performed at 120 °C with 5 mol % [Pd(allyl)Cl]2, 20 mol % dtbpf, and 2 equiv Cy2NMe as base.
