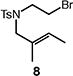
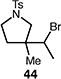
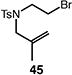
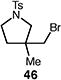
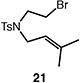
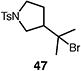
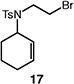
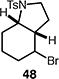
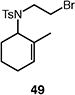
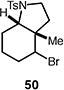
Table 4.
Palladium-catalyzed atom-transfer radical cyclizations of unactivated alkyl bromides.
Reactions performed with [substrate]0 = 0.25 M in PhCF3 at 80 °C with 5 mol % [Pd(allyl)Cl]2, 20 mol % dtbpf, and 0.5 equiv Et3N as base.
Isolated yield.
Yield determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as internal standard.