Table 6.
Comparing the dehydrohalogenation of alkyl bromide and iodide ATRC intermediates.
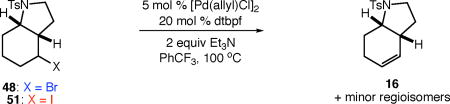
| |||
|---|---|---|---|
|
| |||
| entry | conditions | conversion (%)a | yield (%)a |
| 1 | X = Br, no additive | 100 | 100 |
| 2 | X = I, no additive | 100 | 97 |
| 3 | X = Br, with 10 mol % dinitrobenzene | 22 | 9 |
| 4 | X = I, with 10 mol % dinitrobenzene | 100 | 100 |
| 5 | X = Br, no metal or ligand | <2 | <2 |
| 6 | X = I, no metal or ligand | 56 | 37 |
Determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as internal standard. For details regarding the distribution of minor regioisomers, see Supporting Information.
