Fig 3.
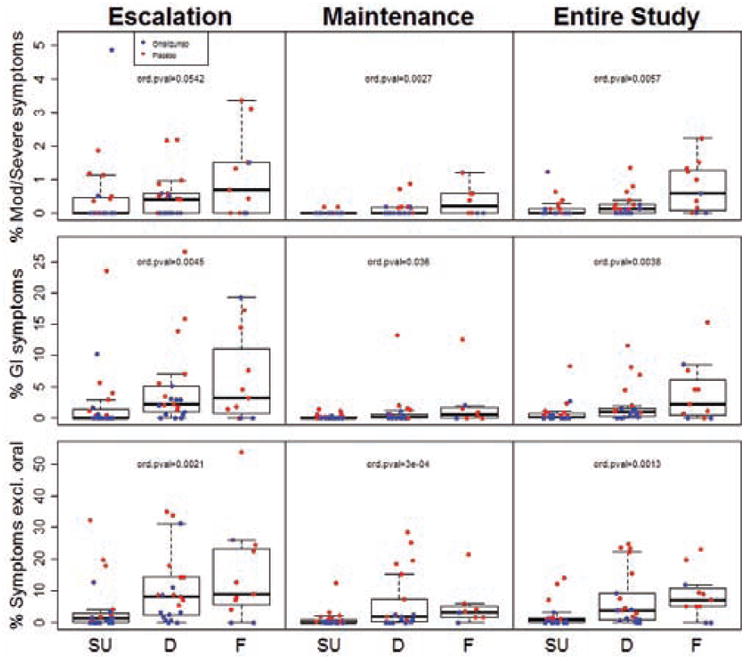
Percent of MOIT doses given during the escalation or maintenance phase of MOIT, or over the course of the entire study, that led to moderate-severe (Mod/Severe) symptoms, Gl symptoms, or any symptom excluding oropharyngeal (Symptoms excl. oral) in subjects who achieved sustained unresponsiveness (SU), were desensitized only (D), or failed the M28 desensitization OFC (F). P-values for a difference in ordinal outcomes (SU/D/F; ord. pval) are presented.
