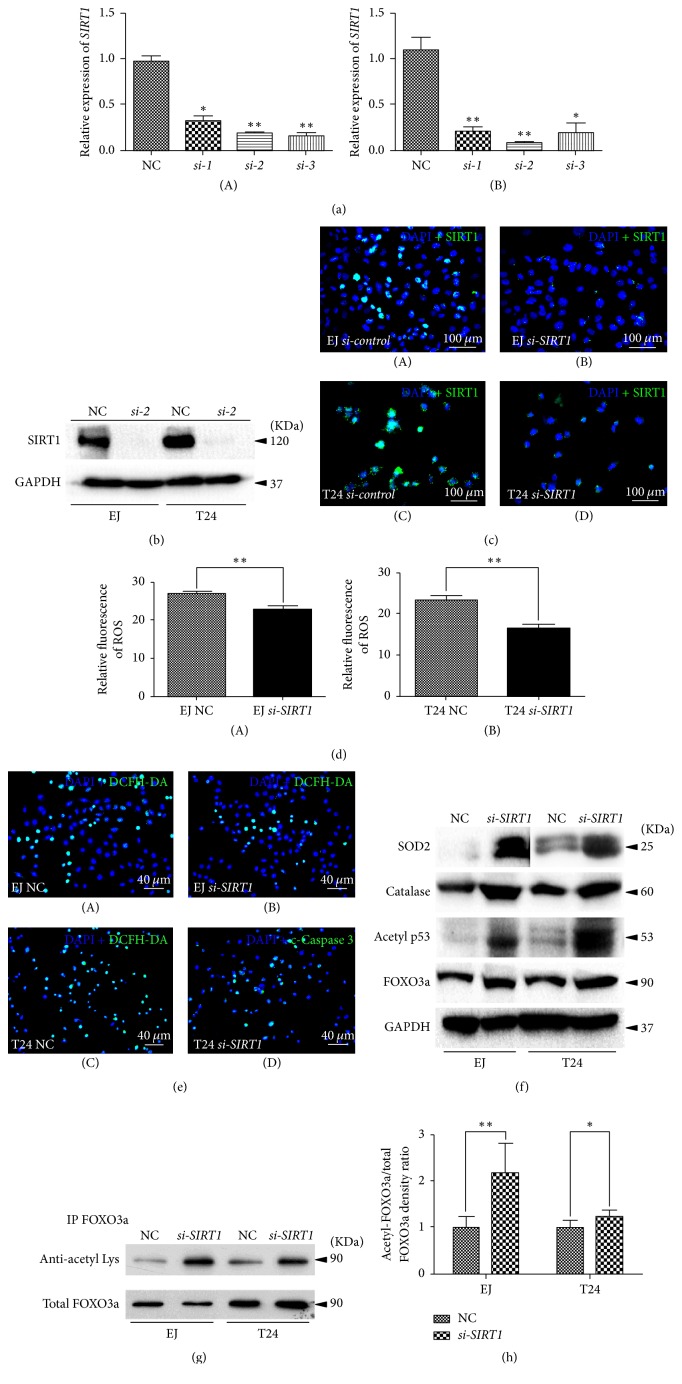
Figure 2.
Knockdown of SIRT1 in BCa cells induced alteration of ROS and related proteins. (a) qRT-PCR was used to testify the KD efficiency by using different siRNA to knockdown SIRT1 in T24 (A) and EJ (B) (contaminated by T24 as per “http://iclac.org/databases/cross-contaminations/”) bladder cancer cells. All shown values were mean ± SD of three measurements and repeated three or more times, ∗p < 0.05 and ∗∗p < 0.01. (b) Western blot bands of SIRT1 and GAPDH in EJ (contaminated by T24 as per “http://iclac.org/databases/cross-contaminations/”) and T24, SIRT1 deficiency BCa cells compared with NC BCa cells. The GAPDH abundance was used as an internal control. (c) Representative immunofluorescence staining of SIRT1 (green) in the BCa cells after SIRT1-target-specific-siRNA treatment (KD) (B, D), compared with control-siRNA treatment (NC) (A, C). Nuclei (blue) were stained by DAPI. The scale bar for (c) is 100 μm. (d) Statistical analysis of relative fluorescence of ROS in the NC and si-SIRT1 transfected EJ (A) (contaminated by T24 as per “http://iclac.org/databases/cross-contaminations/”) and T24 (B) cells. ∗∗p < 0.01. (e) Representative DCFH-DA staining of ROS (green) in the BCa cells with si-SIRT1 treatment (B, D) versus control-siRNA treatment (NC) (A, C). Nuclei (blue) were stained by DAPI. The scale bar for (e) is 40 μm. (f) Western blot analyses of antioxidant enzymes (SOD2, Catalase) acetylated p53 and total FOXO3a in the NC and si-SIRT1 transfected EJ (contaminated by T24 as per “http://iclac.org/databases/cross-contaminations/”) and T24 cells (cell types, siRNA treatment, and protein masses were indicated). The GAPDH was used as a loading control. 10–30 μg of total protein were loaded per lane. (g) Immunoprecipitation analysis of antiacetylated Lysine and total FOXO3a in the NC and si-SIRT1 transfected EJ (contaminated by T24 as per “http://iclac.org/databases/cross-contaminations/”) and T24 cells. (h) Statistical analysis of relative acetylation rate of FOXO3a by measurement of the band intensity of three independent Western blot experiments, calculated as relative acetylation rate of FOXO3a = intensity of antiacetylated Lysine/intensity of total FOXO3a. The intensity of total FOXO3a was used as control and its rate of NC group was normalized to 1. ∗p < 0.05; ∗∗p < 0.01.