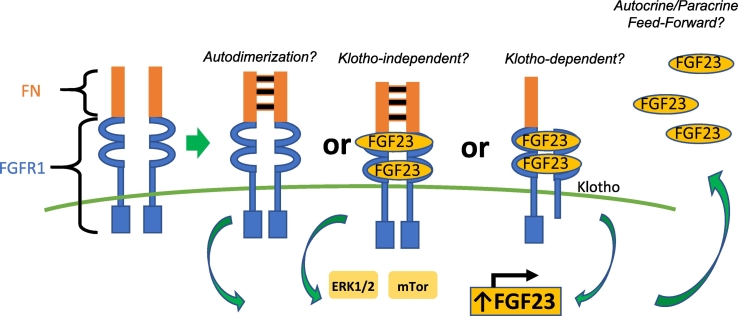
Fig. 6.
Fibronectin-fibroblast growth factor receptor 1 translocations in TIO.
Depicted is the putative chimeric protein generated by the fibronectin-fibroblast growth factor 1 (FN1-FGFR1) translocations that were identified in a subset of the phosphaturic mesenchymal tumors that cause tumor-induced osteomalacia (Berglund et al., 2017, Lee et al., 2015, Lee et al., 2016). The chimeric protein includes the fibronectin extracellular autodimerization domain and the FGFR1 ligand binding, transmembrane and intracellular tyrosine kinase signaling domain. As such, the receptor has the potential to autodimerize, resulting in ligand independent signaling. It can also dimerize after FGF23 binding, thus signaling in a ligand-dependent fashion. Transduction continues through the extracellular signal–regulated kinase pathway (ERK1/2) and/or the mechanistic target of rapamycin (mTOR) pathway. If Klotho is also expressed by PMTs, it is possible that FGF23 could bind and signal in a Klotho-dependent manner. This signaling could lead to increased transcription, translation, and secretion of FGF23 by tumor cells via a feed-forward system. (Figure modified by Jason Berglund) (Minisola et al., 2017).