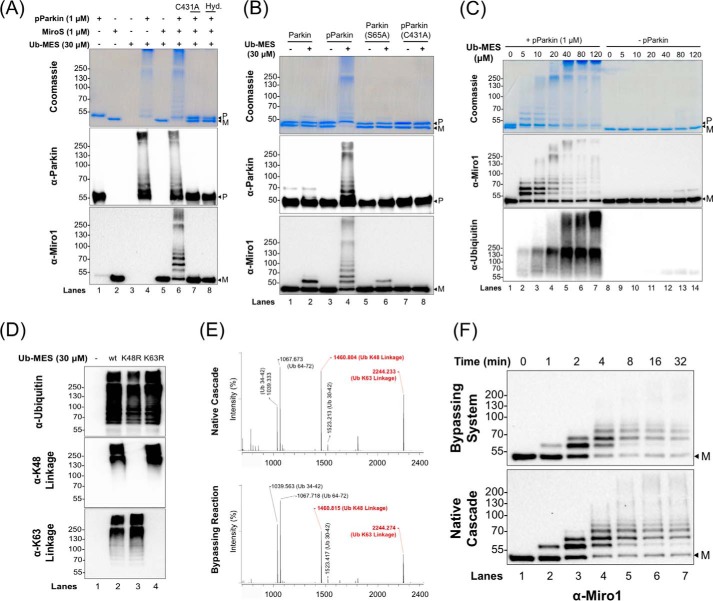
Figure 2.
PARKIN/ByS recapitulates several aspects of the native cascade. A, ByS reaction of pPARKIN, UbMES, and the MiroS substrate. Lane 4 shows pPARKIN autoubiquitination and chain formation, and lane 6 shows pPARKIN autoubiquitination, chain formation, and MiroS substrate ubiquitination. Notably, UbMES has no ubiquitination activity in the absence of pPARKIN or in the presence of catalytically inactive C431A pPARKIN (lanes 5 and 7). Hyd. indicates hydrolyzed UbMES before the reaction. B, pPARKIN undergoes autoubiquitination and MiroS ubiquitination using UbMES. Mutation of the primary phosphorylation site (S65A) or the catalytic cysteine (C431A) blocks this activity. P indicates PARKIN; M indicates MiroS, and * indicates impurities. PARKIN (S65A) was pretreated with TcPINK1 under the same reaction conditions as PARKIN, prior to its use in assay. C, ByS ubiquitination depends on the concentration of UbMES and requires pPARKIN. D, pPARKIN/ByS forms Lys48- and Lys63-linked chains, similar to the native cascade. The reactions with pPARKIN, MiroS, and the corresponding UbMES were analyzed by Western blot. A K48R mutation in UbMES blocks formation of Lys48-linked chains but not Lys63-linked chains (lane 3), whereas a K63R mutation in UbMES blocks formation of Lys63-linked chains but not Lys48-linked chains (lane 4). E, MALDI-TOF analysis of a slice from the stacking gel of a native cascade reaction and the ByS reaction from D, lane 2, confirming Lys48 and Lys63 chain formation. F, MiroS ubiquitination by pPARKIN using the ByS (1 μm pPARKIN and 30 μm UbMES) proceeds on a similar time scale to common native reaction conditions (100 nm UBE1, 1 μm UbcH7, 1 μm pPARKIN, 30 μm Ub, and 4 mm ATP). P indicates PARKIN, and M indicates MiroS.