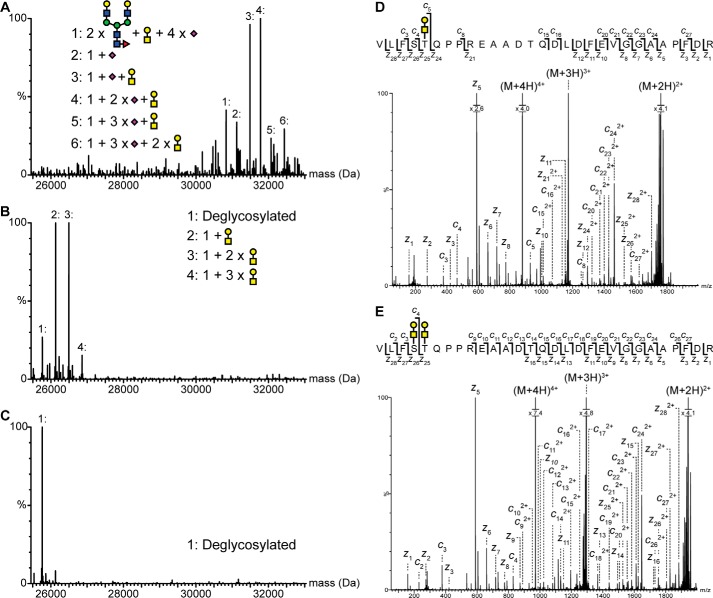
Figure 2.
Identification and mapping of N- and O-linked glycans in the pro-part of proNGF. Intact mass analysis of nontreated (A), PNGase- and sialidase-treated (B), and PNGase F-, sialidase-, and O-glycosidase-treated (C) proNGF. Treatment with both PNGase F and O-glycosidase caused a mass shift corresponding to known glycan structures, showing that proNGF is both N- and O-linked glycosylated. The ETD fragmentation spectra of glycopeptide Val−35–Arg−7 with either two (E) or one (D) O-linked glycan show that proNGF is O-linked glycosylated at Ser−32 and Thr−31. The mass error of all identified peaks is below 23 ppm. Yellow square, N-acetylgalactosamine; blue square, N-acetylglucosamine; yellow circle, galactose; green circle, mannose; purple diamond, sialic acid; red triangle, fucose.