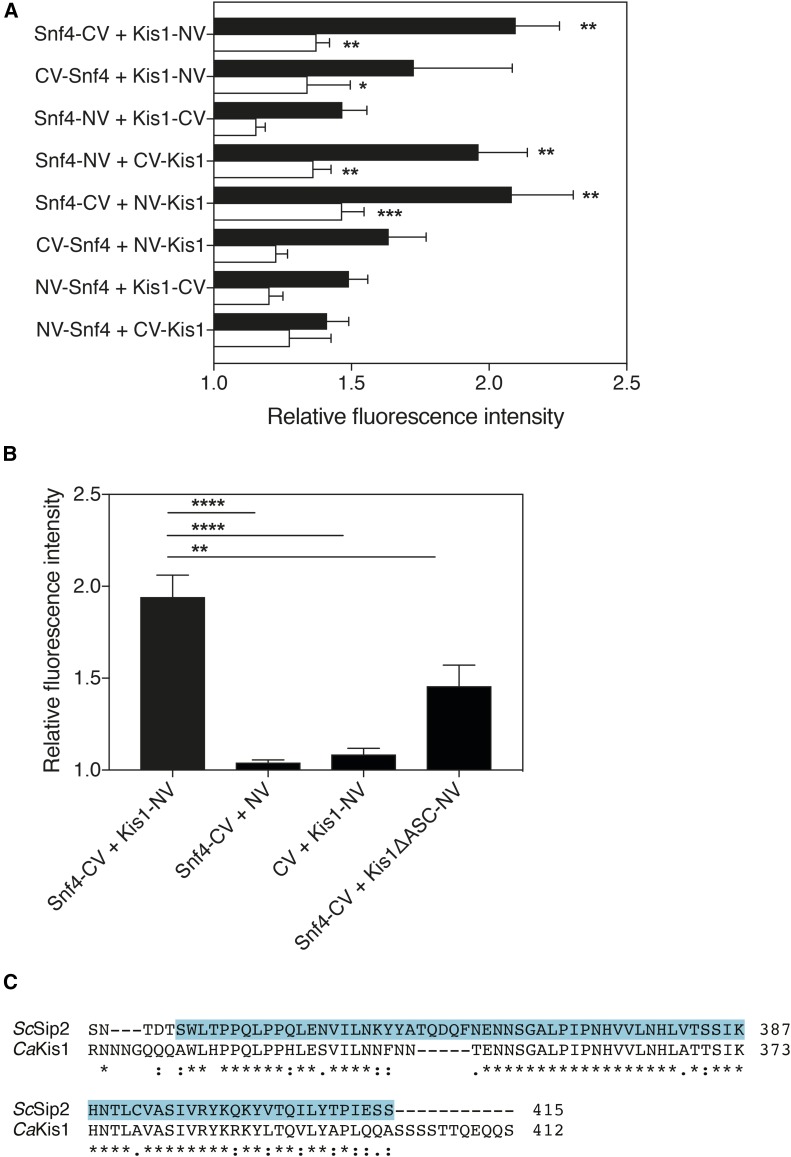
Figure 2.
(A) Microplate reader analysis of all eight Snf4-Kis1 BiFC combinations. Quantitative analysis of Snf4-Kis1 BiFC signal in the exponential phase (black bars) and stationary phase (white bars). Relative fluorescence intensity represents the ratio of BiFC strains’ fluorescence over fluorescence of background strain containing backbone vectors. Data represent mean values ± SEM (n = 5). The experiment was performed twice with similar results; a representative experiment is shown. *Significant difference compared with the background strain determined in the same growth phase (two-way ANOVA, Sidak’s multiple comparisons test, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001). (B) Microplate reader analysis of the optimal Snf4-Kis1 BiFC combination by including the negative controls. Snf4-C-yEmVenus and Kis1-N-yEmVenus both tagged C-terminally were selected as the optimal combination. Kis1ΔASC shows significantly less fluorescence complementation with Snf4 compared to the full-length Kis1. Fluorescence was analyzed by the microplate system, in the exponential phase of growth. Relative fluorescence intensity represents the ratio of BiFC strains’ fluorescence over fluorescence of background strain containing backbone vectors. Data represent mean values ± SEM (n = 5). The experiment was performed twice with similar results; a representative experiment is shown. *Significant difference compared to control strains (one-way ANOVA, Dunnett’s multiple comparisons test, ** P ≤ 0.01, **** P ≤ 0.0001). (C) Alignment of the C. albicans Kis1 and homologous Sip2 protein in S. cerevisiae. The identified ScSip2 ASC domain (Jiang and Carlson 1997) is highlighted in blue. Alignment was performed using the Clustal Omega online tool.