Abstract
Objectives
Based on data from Chinese and Indian traditional herbal medicines, gum resin of Ferula assa-foetida (sometimes referred to asafetida or asafoetida) has several therapeutic applications. The authors of various studies have claimed that asafetida has cytotoxic, antiulcer, anti-neoplasm, anti-cancer, and anti-oxidative effects. In present study, the anti-aging effect of asafetida on senescent human dermal fibroblasts was evaluated.
Methods
Senescence was induced in in vitro cultured human dermal fibroblasts (HDFs) through exposure to H2O2, and the incidence of senescence was recognized by using cytochemical staining for the activity of β-galactosidase. Then, treatment with oleo gum resin of asafetida was started to evaluate its rejuvenating effect. The survival rate of fibroblasts was evaluated by using methyl tetrazolium bromide (MTT) assays. Real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blot assays were performed to evaluate the expressions of apoptotic and anti-apoptotic markers.
Results
Our experiments show that asafetida in concentrations ranging from 5 × 10−8 to 10−7 g/mL has revitalizing effects on senescent fibroblasts and significantly reduces the β-galactosidase activity in these cells (P < 0.05). Likewise, treatment at these concentrations increases the proliferation rate of normal fibroblasts (P < 0.05). However, at concentrations higher than 5 × 10−7 g/mL, asafetida is toxic for cells and induces cell death.
Conclusion
The results of this study indicate that asafetida at low concentrations has a rejuvenating effect on senescent fibroblasts whereas at higher concentrations, it has the opposite effect of facilitating cellular apoptosis and death.
Keywords: antioxidants, cell senescence, fibroblasts, oxidative stress, rejuvenation
1. Introduction
Consumption of medicinal herbs is growing and people want to reduce the practice of using chemically synthesized drugs [1, 2]. Despite this growing interest for herbal medicine, our knowledge of their possible benefits or adverse side effects is not sufficient [3]. Part of this lack of information arises from the complexity and the diversity of each plant’s constituents, and the other part is the fact that each constituent can exert various effects on the body’s organs [2]. One such medicinal herb is Ferula assa-foetida, which belongs to the umbelliferae family of plants; several therapeutic applications, such as anti-diabetic, anti-ulcer, aphrodisiac, antiepileptic, anthelmintic, and antispasmodic applications, have been proposed for this herb [4]. The oleo-gum-resin of Ferula assa-foetida is sometimes referred to as asafetida or asafoetida, but for consistency asafetida will be used throughout this paper.
A review of the compounds in asafetida shows that some skin-friendly compounds, as well as some irritant substances, are contained in its gum resin. One of these skin-friendly compounds which has a known antioxidative effect is ferulic acid (FA) [5]. Several reports about the biological roles of FA have been published, and most are about its antioxidative properties [6]. FA protects skin from UV radiation by forming a resonance-stabilized phenoxy radical and by preventing the activation of caspase in dermal fibroblasts [7–9]. It is a potent antioxidant and synergizes the effects of ascorbic acid [10]; it also protects cells from oxidative damage by neutralizing different species of free radicals, such as hydroxyls, alkoxyls, peroxyls, nitric oxide, peroxynitrites, and superoxides [10–17]. In addition to these reported properties, some evidence exists regarding the anti-mutagenicity of FA, indicating that it protects cells from menadione-induced oxidative DNA damage; its anti-carcinogenic effects have also been demonstrated in animal models of pulmonary and colon cancers [18–23]. In experiments regarding its dermal application, FA decreased UVB-induced erythema, 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ornithine decarboxylase activity, and TPA-induced skin tumor formation [24, 25].
Apart from FA, some researchers have found that asafetida contains some other ingredients that can cause or inhibit skin irritation. For example, it contains alpha pinene with its reported anti-inflammatory and analgesic effects [26] and alpha terpineol with its reported anti-inflammatory and skin-irritating effects [27]. It also contains other compounds, such as diallyl disulfide, luteolin, and isopimpinellin with their confirmed capabilities for the prevention of chemically-induced skin tumor development in mice [28–31], as well as Azulene [4], which is beneficial for the prevention of skin irritation and skin damage and is widely used in cosmetic products [32].
Based on the above data, asafetida contains many compounds that can affect skin cells, and most of them have or may have therapeutic applications for skin problems such as aging. Until now, to the best of our knowledge, no report on the effect of asafetida extract on skin cells has been reported. For that reason, we designed this study to evaluate the effect of asafetida on normal and senescent human dermal fibroblasts (HDFs). To evaluate the effect of asafetida on HDFs, we used a reactive oxygen species (ROS)-mediated model of senescence; consequently, we selected, some important regulators of the ROS-mediated apoptosis pathway, which are listed in Table 1, for further analysis. BCL2, BAD and BAX are apoptosis regulators acting on the mitochondrial membrane [33, 34], and p21 is the main downstream regulator of p53-dependent cell cycle arrest and senescence in response to DNA damage [35]. CASP3 was selected because its activation could be triggered by both extrinsic (death ligand) and intrinsic (mitochondrial) pathways [36]. ALOX5 was selected so that we could evaluate the inflammatory responses of senescent HDFs after treatment with asafetida [37].
Table 1.
List of the primers used for the real-time qRT-PCR assay
| Gene | Primer sequences | GenBank |
|---|---|---|
| ACTB | F: AGTTGCGTTACACCCTTTCTT | NM_001101.3 |
| R: CACCTTCACCGTTCCAGTTT | ||
|
| ||
| p21 | F: GGTGTGTGCTGCGTTCA | NM_001220778.1 |
| R: AAGTTCCATCGCTCACGG | ||
|
| ||
| BAX | F: CAGGGTGGTTGGGTGAGAC | NM_001291430.1 |
| R: TGAAGATGGGGAGAGGGCA | ||
|
| ||
| BAD | F: CTCCACATCCCGAAACTCCA | NM_032989.2 |
| R: GTCAGCCCTCCCTCCAAAG | ||
|
| ||
| BCL2 | F: TGTGTGGAGAGCGTCAACC | NM_000633.2 |
| R: CTTCAGAGACAGCCAGGAGAA | ||
|
| ||
| CASP3 | F: GCCTGTAACTTGAGAGTAGATGGT | NM_032991 |
| R: TGCGTATGGAGAAATGGGCT | ||
|
| ||
| ALOX5 | F: GACCTGACCTATGCCTCCCT | NM_000698.3 |
| R: GTCCTGATGGCTTCCCACAC | ||
qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
2. Materials and Methods
Oleo-gum resin of Ferula assa-foetida L. (asafetida) was prepared based on our previous study [38]. In our experiments, we used a special, pure type of asafetida (Ashki asafetida in local dialect) that, based on high performance liquid chromatography (HPLC) assays, has a higher concentration of FA [38]. Briefly, Oleo-gum resin was collected in June from Ferula assa-foetida L. (Herbarium code: P1006636, IBRC, Tehran, Iran) by making some small incisions (1 – 5 cm) on its stem near to its roots, from which high-quality oleo-gum resin (asafetida) could be obtained. The collected asafetida was cut into small pieces and placed under a hood until it had dried. For preparation of the asafetida solution, a specified amount (10 mg) of dried asafetida was dissolved in 10 mL of distilled water and filtered by using 0.02-um filters. Different volumes of this asafetida solution were added to the culture media of cells to obtain the final treatment concentrations.
A HDF cell line (NCBI Code: C646, Pasteur Institute, Tehran, Iran), cell passage numbers 5 – 8, was used for cell assessments. According to the manufacturer’s data, this cell line was derived from the dermis of normal human neonatal foreskin and cryopreserved at the end of primary culture; a cell passage number less than 10 is safe and cytogenetically stable. Cells were cultured in DMEM medium containing 10% FBS (2 × 104 cells/well of 24-well plates for MTT and staining assays and 106 cells/well of 6-well plates for real time qRT-PCR assays). The effects of asafetida on normal and senescent HDFs were evaluated after 10 days of treatment with different doses of asafetida (10−8, 5 × 10−8, 10−7, 5 × 10−7 and 10−6 g/mL).
ROS-mediated senescence was induced on HDFs by using hydrogen peroxide [39]. For this purpose, passage-four HDFs were cultured in 24-well culture plates at a density of 2 × 104/well. The following day, the medium was replaced with a medium containing 600-μM H2O2 (hydrogen peroxide), after which the plates were placed in a CO2 incubator for 2 hours. Next, the medium was replaced with a normal fibroblast medium (DMEM + 10% FBS). After the cells had been incubated in the CO2 incubator for 24 hours, they were exposed for the second time to 600-μM H2O2. Two hours later, the medium was replaced with normal fibroblast medium containing different concentrations of asafetida extract. The culture medium was changed every 3 days while fresh asafetida was added to the medium daily for 10 days. The final concentrations of asafetida were 0 (non-treated, control), 10−8, 5 × 10−8, 10−7, 5 × 10−7, and 10−6 g/mL of gum resin of asafetida dissolved in the culture medium. Each treatment was repeated in four replicates.
After 10 days of treatment with asafetida, the density of senescent cells was measured by staining the senescent cells. Cell staining was performed by using Senescence Cells Histochemical Staining Kits (Sigma, CS0030). The assay is based on a cytochemical stain for β-galactosidase activity at pH 6. After staining, 20 images with 40 x magnification were captured from each treated group, and the numbers of senescent cells were counted in each image. Cell viability was measured using the 3-(4, 5-dimethyl-2thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT, Sigma) assay. This method is based on the mitochondrial dehydrogenase activity of vivid cells in the culture dish. For this purpose, cells belonging to each treatment group were cultured in one 24-well culture dish; then, two days after initiation of asafetida treatment, 50 μL of MTT (5 mg/mL) were added to the culture medium, and the cells were incubated for 4 hours. After that, the culture medium was removed completely and replaced with 250 μL of DMSO. The absorbance of each well was measured at 560 nm by using a spectrophotometer, and the results were shown as the percent of each group compared to the control group.
Real time PCR was performed on senescent HDFs to measure the expression rates of the apoptotic markers p21, BAX, BAD, and caspase 3 (CASP3), the antiapoptotic marker BCL2, and an inflammatory marker ALOX5 (Arachidonate 5-lipoxygenase). β-actin mRNA (ACTB) was used as a housekeeping gene. The list of primers sequences is presented in Table 1. After isolation of total RNA (Vivantis RNA isolation Kit), CDNAs were synthesized using MMLV Reverse Transcriptase and Oligo (dT) Primers according to the manufacturer’s instructions (Vivantis, Easy cDNA reverse transcription kit). For gene expression analysis, relative quantitation PCR (qPCR) was performed using SYBR-Green master mix (Qiagen) in a Qiagen Rotor Gene 6000 system and software. The qPCR conditions were 1 cycle of incubation in 94°C for 10 min for denaturation, and then DNA amplification was performed in 40 cycles using 1 min in 53°C for annealing, 20 seconds in 72°C for elongation, and 15 seconds in 95°C for denaturation. The expression levels of these target genes in each sample were calculated by using the comparative Ct method (2−ΔΔCt formula), after having been normalized by the Ct value of the housekeeping gene in each group. All experiments were repeated three times for each group.
For the Western blot analyses, cells were lysed on ice in a lysis buffer containing 20-mM Tris-HCl (pH 7.5), 150-mM NaCl, 10-mM EDTA (pH 7.5), 1% Triton X-100, and 1% deoxycholate. Then, the cell extracts were clarified by centrifugation, resolved on SDS-PAGE, and transferred onto PVDF membranes (Millipore, USA). After having been blocked with 5% BSA overnight at 4°C, the membranes were incubated for 1.5 h at room temperature with rabbit primary anti-BCL-2 antibody (Abcam, ab59348, 1 ː 500) and mouse primary anti-GAPDH antibody (Abcam, ab8245, 1: 1000). Following three rinses (15 min each) with PBS-Tween20 (0.05%), incubation with the peroxidase (HRP)-conjugated goat anti-rabbit IgG H&L (Abcam, ab205718, 1:2000) and goat anti mouse secondary antibody (Abcam, ab97240, 1ː2000) was performed for 2 hours at RT. After three washes with TBST, western blotting chemiluminescence reagent (Thermo Scientific, USA) was used for protein detection. The relative intensities of western blot bands were semi-quantified by using Image J software. The relative band intensity for each protein was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical analyses were performed using SPSS software. Each experiment was repeated a minimum of three times, and the data were expressed as means ± standard errors of the mean (SEMs). Statistical differences between the groups were assessed by using the one-way analysis of variance (ANOVA) followed by Tukey’s test. Statistical significance was established at P < 0.05.
3. Results
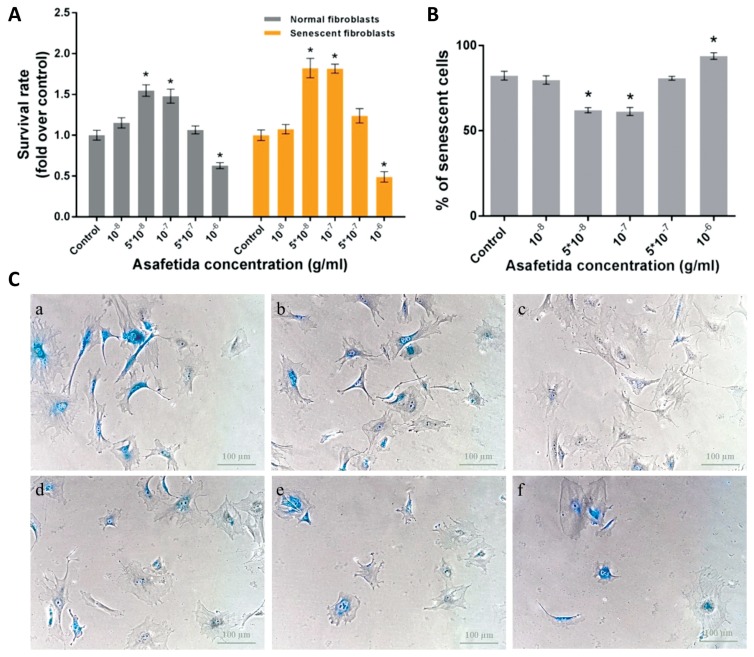
The results of the MTT assays showed that treatments with asafetida at concentrations of 5 × 10−8 and 10−7 g/mL could increase the survival rates of normal and senescent HDFs compared with the other groups (P < 0.05, Fig. 1A). To evaluate the anti-aging effect of asafetida, we treated senescent cells with different concentrations of asafetida for 10 days and then measured the density of senescent cells by using β-galactosidase staining. The mean percentage of senescent cells per total number of cells showed that the numbers of senescent cells in the groups treated at concentration of 5 × 10−8 and 10−7 g/mL were significantly lower than the numbers of such cells in the other groups (P < 0.05, Fig. 1). Our data also showed that asafetida was toxic at higher concentrations (10−6 g/mL) and that it significantly reduced the survival rate of HDFs and subsequently increased the number of senescent cells (P < 0.05, Fig. 1).
Figure 1.
(A) MTT assay results in normal and senescent fibroblasts (*P < 0.05). (B) Effect of treatment with asafetida on human senescent fibroblasts in percent of senescent cells/total number of cells counted in the images from each group (*P < 0.05). (C) Images of cells after staining for β-galactosidase activity, with the blue color representing senescent cells: (a) control, (b) 10−8, (c) 5 × 10−8, (d) 10−7, (e) 5 × 10−7, and (f) 10−6 g/mL of asafetida. Data are presented as means ± SEMs, and scale bars represent 100 μm.
MTT, methyl tetrazolium bromide; SEMs, standard errors of the mean.
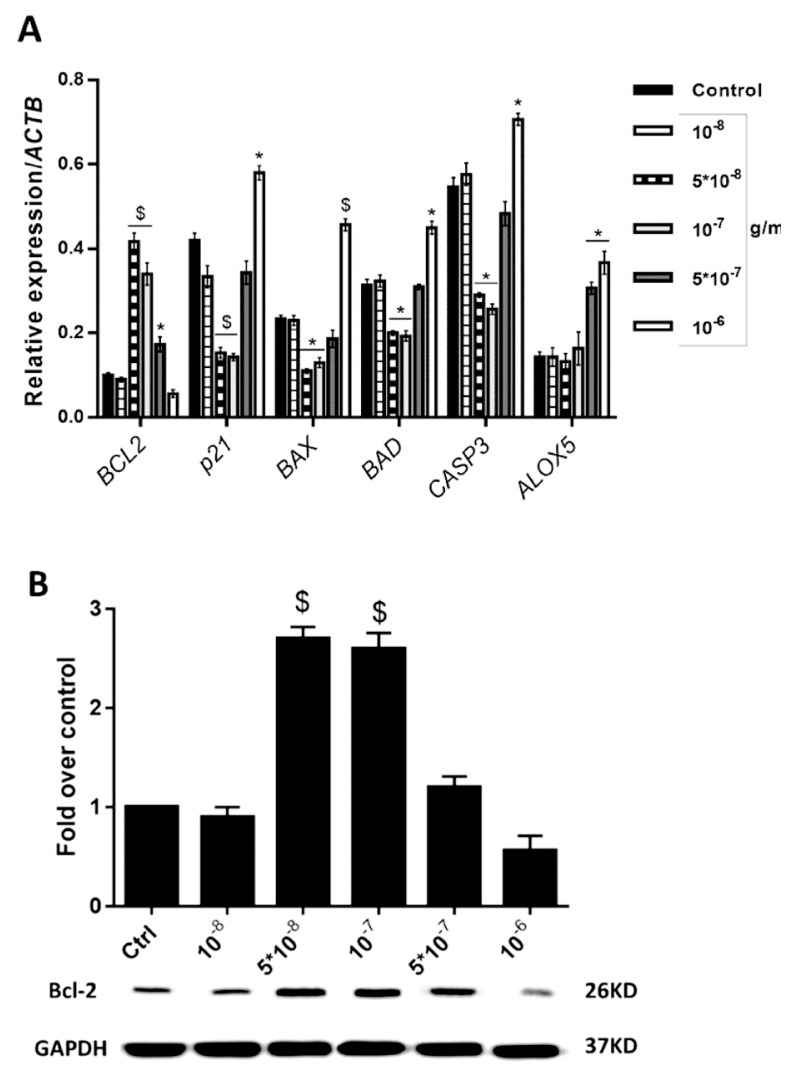
As shown in Fig. 2A, gene expression assays revealed that treatments with 5 × 10−8 and 10−7 g/mL of asafetida increased the expression of BCL2 (P < 0.01), but decreased the expressions of p21, CASP3, BAX and BAD as compared with the control group (P < 0.01 for p21 and CASP3; P < 0.05 for BAD and BAX). The expression level of ALOX5 revealed no change at these concentrations as compared with the control group. Otherwise, for doses above 10−7 g/mL, asafetida prompted apoptotic function, and the expression rate of anti-apoptotic BCL2 was significantly decreased to very low levels as compared with the control group (P < 0.05); however, the expressions of the apoptotic inducer factors BAX, BAD, CASP3 and p21 were increased (P < 0.05). The expression of ALOX5 was also increased, as compared with the control group, in the groups that were treated with higher doses of asafetida (P < 0.01). Results of western blot analyses also confirmed an approximately threefold increase in BCL2 protein expression in groups treated at asafetida concentrations of 5 × 10−8 and 10−7 g/mL as compared with the other groups (P < 0.01, Fig. 2B).
Figure 2.
(A) Expression levels of apoptotic and anti-apoptotic mRNAs in asafetida-treated senescent cells. The expression of BCL2 was increased in the groups treated with 5 × 10−8 and 10−7 g/mL of asafetida compared with the control group (P < 0.01). Also, in those groups, the expression rates of apoptotic markers were significantly reduced as compared with the control group while in the groups treated with 5 × 10−7 and 10−6 g/mL of asafetida, the expression rates of apoptotic markers were increased. (B) Western blot assay for BCL2 protein in different groups (*P < 0.05 and $P < 0.01, compared with the control group).
4. Discussion
In present study, we measured the effects of water soluble parts of asafetida (gum resin of Ferula assa-foetida) on human dermal fibroblasts. Asafetida has multiple components, and among them, FA is a well-known compound because of its anti-apoptotic effects. Plants of the ferula species also have high amounts of sulfide compounds and rare concentrations of sesquiterpene coumarins and terpenes with anti-inflammatory effects [40–42]. The results of MTT and β-galactosidase staining assays showed that treatments with low concentrations of asafetida could protect senescent HDFs from apoptosis. To evaluate this effect at a molecular level, we performed real-time qRT-PCR and western blot assays, and the results showed that treatment with asafetida altered the expression rates of apoptotic and anti-apoptotic markers in fibroblasts. Reductions in the expressions of p21 (anti-proliferative and senescence-inducing factor) [43], CASP3, BAX, and BAD (mediators of programmed cell death), as well as surges in the expression of BCL2 (anti-apoptotic marker) [44], revealed that asafetida had a potent anti-apoptotic effect. Furthermore, the results of cell staining for beta-galactosidase activity confirmed that these reductions of BAX and BAD (as positive regulators of cell apoptosis) and the increase in BCL2 effectively rejuvenated senescent fibroblasts.
Based on the recent findings about the roles of 5-lipoxigenase (ALOX5, 5-LO) in the activation of pro-inflammatory pathways [37], we also evaluated the expression of ALOX5 in our experiments. When the treatment dosage of asafetida was increased above 10−7 g/mL, the expression level of ALOX5 was increased in cells, which revealed that asafetida could activate pro-inflammatory signals. In confirmation, one report indicated that topical administration of asafetida could cause contact dermatitis in infants [45]. This skin-irritating effect of asafetida might be due to its disulfide-containing compounds [46]. Thus, this pro-inflammatory side effect of asafetida should be diminished by reducing the concentration of its sulfide compounds through chemical processing or by combining it with ALOX-5 inhibitory herbal supplements, such as an extract of Tripterygium wilfordii [47].
The results of the present study demonstrate that asafetida has both apoptotic and anti-apoptotic effects. In optimal doses, it reverses senescence, but has the opposite effect at higher concentrations. Moreover, toxic concentrations of asafetida can be useful for skin exfoliation. Nevertheless, further studies are needed to identify its efficacy in vivo.
5. Conclusion
The results of the present study revealed that asafetida in low concentrations had an anti-senescence effect on human dermal fibroblasts. This effect was due to its role on enhancing the expression of the anti-apoptotic factor BCL2.
Acknowledgment
This study was equally supported by the Neurobiomedical Research Center (NRC), Yazd, Iran, and Royan Institute, Tehran, Iran.
Footnotes
This paper meets the requirements of KS X ISO 9706, ISO 9706-1994 and ANSI/NISO Z39.48-1992 (Permanence of Paper).
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2013;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan SY, Litscher G, Gao SH, Zhou SF, Yu ZL, Chen HQ, et al. Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/525340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Lorenzo C, Ceschi A, Kupferschmidt H, Lüde S, De Souza Nascimento E, Dos Santos A, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578–92. doi: 10.1111/bcp.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahendra P, Bisht S. Ferula asafoetida: traditional uses and pharmacological activity. Pharmacogn Rev. 2012;6(12):141–6. doi: 10.4103/0973-7847.99948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kareparamban JA, Nikam PH, Jadhav AP, Kadam VJ. A validated high-performance liquid chromatograhy method for estimation of ferulic acid in asafoetida and polyherbal preparation. Indian J Pharm Sci. 2013;75(4):493–5. doi: 10.4103/0250-474X.119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiao Y, He H, Zhang Z, Liao Z, Yin D, Liu D, et al. Long-term sodium ferulate supplementation scavenges oxygen radicals and reverses liver damage induced by iron overloading. Molecules. 2016;21(9):E1219. doi: 10.3390/molecules21091219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn HJ, Kim KB, Bae S, Choi BG, An S, Ahn KJ, et al. Pretreatment of ferulic acid protects human dermal fibroblasts against ultraviolet a irradiation. Ann Dermatol. 2016;28(6):740–8. doi: 10.5021/ad.2016.28.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouimet MA, Faig JJ, Yu W, Uhrich KE. Ferulic acid-based polymers with glycol functionality as a versatile platform for topical applications. Biomacromolecules. 2015;16(9):2911–9. doi: 10.1021/acs.biomac.5b00824. [DOI] [PubMed] [Google Scholar]
- 9.Graf E. Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992;13(4):435–48. doi: 10.1016/0891-5849(92)90184-I. [DOI] [PubMed] [Google Scholar]
- 10.Trombino S, Serini S, Di Nicuolo F, Celleno L, Ando S, Picci N, et al. Antioxidant effect of ferulic acid in isolated membranes and intact cells: synergistic interactions with alpha-tocopherol, beta-carotene, and ascorbic acid. J Agric Food Chem. 2004;52(8):2411–20. doi: 10.1021/jf0303924. [DOI] [PubMed] [Google Scholar]
- 11.Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002;50(7):2161–8. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- 12.Kaul A, Khanduja KL. Polyphenols inhibit promotional phase of tumorigenesis: relevance of superoxide radicals. Nutr Cancer. 1998;32(2):81–5. doi: 10.1080/01635589809514723. [DOI] [PubMed] [Google Scholar]
- 13.Hynes MJ, O’Coinceanainn M. The kinetics and mechanisms of reactions of iron(III) with caffeic acid, chlorogenic acid, sinapic acid, ferulic acid and naringin. J Inorg Biochem. 2004;98(8):1457–64. doi: 10.1016/j.jinorgbio.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Wenk GL, McGann-Gramling K, Hauss-Wegrzyniak B, Ronchetti D, Maucci R, Rosi S, et al. Attenuation of chronic neuroinflammation by a nitric oxide-releasing derivative of the antioxidant ferulic acid. J Neurochem. 2004;89(2):484–93. doi: 10.1111/j.1471-4159.2004.02359.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogiwara T, Satoh K, Kadoma Y, Murakami Y, Unten S, Atsumi T, et al. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2002;22(5):2711–7. [PubMed] [Google Scholar]
- 16.Pannala AS, Razaq R, Halliwell B, Singh S, Rice-Evans CA. Inhibition of peroxynitrite dependent tyrosine nitration by hydroxycinnamates: nitration or electron donation? Free Radic Biol Med. 1998;24(4):594–606. doi: 10.1016/S0891-5849(97)00321-3. [DOI] [PubMed] [Google Scholar]
- 17.Dinis TC, Santosa CL, Almeida LM. The apoprotein is the preferential target for peroxynitrite-induced LDL damage protection by dietary phenolic acids. Free Radic Res. 2002;36(5):531–43. doi: 10.1080/10715760290025915. [DOI] [PubMed] [Google Scholar]
- 18.Yamada J, Tomita Y. Antimutagenic activity of caffeic acid and related compounds. Biosci Biotechnol Biochem. 1996;60(2):328–9. doi: 10.1271/bbb.60.328. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson LR, Lim IF, Pearson AE, Ralph J, Harris PJ. Bacterial antimutagenesis by hydroxycinnamic acids from plant cell walls. Mutat Res. 2003;542(1–2):49–58. doi: 10.1016/j.mrgentox.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Lesca P. Protective effects of ellagic acid and other plant phenols on benzo[a]pyrene-induced neoplasia in mice. Carcinogenesis. 1983;4(12):1651–3. doi: 10.1093/carcin/4.12.1651. [DOI] [PubMed] [Google Scholar]
- 21.Burdette JE, Chen SN, Lu ZZ, Xu H, White BE, Fabricant DS, et al. Black cohosh (Cimicifuga racemosa L.) protects against menadione-induced DNA damage through scavenging of reactive oxygen species: bioassay-directed isolation and characterization of active principles. J Agric Food Chem. 2002;50(24):7022–8. doi: 10.1021/jf020725h. [DOI] [PubMed] [Google Scholar]
- 22.Kawabata K, Yamamoto T, Hara A, Shimizu M, Yamada Y, Matsunaga K, et al. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157(1):15–21. doi: 10.1016/S0304-3835(00)00461-4. [DOI] [PubMed] [Google Scholar]
- 23.Wargovich MJ, Jimenez A, McKee K, Steele VE, Velasco M, Woods J, et al. Efficacy of potential chemopreventive agents on rat colon aberrant crypt formation and progression. Carcinogenesis. 2000;21(6):1149–55. doi: 10.1093/carcin/21.6.1149. [DOI] [PubMed] [Google Scholar]
- 24.Saija A, Tomaino A, Trombetta D, De Pasquale A, Uccella N, Barbuzzi T, et al. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int J Pharm. 2000;199(1):39–47. doi: 10.1016/S0378-5173(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 25.Huang MT, Smart RC, Wong CQ, Conney AH. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48(21):5941–6. [PubMed] [Google Scholar]
- 26.Gardin C, Piattelli A, Zavan B. Graphene in regenerative medicine: focus on stem cells and neuronal differentiation. Trends Biotechnol. 2016;34(6):435–7. doi: 10.1016/j.tibtech.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Mercier B, Prost J, Prost M. The essential oil of turpentine and its major volatile fraction (alpha- and beta-pinenes): a review. Int J Occup Med Environ Health. 2009;22(4):331–42. doi: 10.2478/v10001-009-0032-5. [DOI] [PubMed] [Google Scholar]
- 28.Shan Y, Wei Z, Tao L, Wang S, Zhang F, Shen C, et al. Prophylaxis of diallyl disulfide on skin carcinogenic model via p21-dependent Nrf2 stabilization. Sci Rep. 2016;6:35676. doi: 10.1038/srep35676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HC, Yang JH, Hsieh SC, Sheen LY. Allyl sulfides inhibit cell growth of skin cancer cells through induction of DNA damage mediated G2/M arrest and apoptosis. J Agric Food Chem. 2010;58(11):7096–103. doi: 10.1021/jf100613x. [DOI] [PubMed] [Google Scholar]
- 30.Palombo R, Savini I, Avigliano L, Madonna S, Cavani A, Albanesi C, et al. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016;7(8):e2344. doi: 10.1038/cddis.2016.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleiner HE, Vulimiri SV, Starost MF, Reed MJ, DiGiovanni J. Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 2002;23(10):1667–75. doi: 10.1093/carcin/23.10.1667. [DOI] [PubMed] [Google Scholar]
- 32.Ueki JI, Sakagami H, Wakabayashi H. Anti-UV activity of newly-synthesized water-soluble azulenes. In vivo. 2013;27(1):119–26. [PubMed] [Google Scholar]
- 33.Hardwick JM, Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol. 2013;5(2) doi: 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17(3):393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, et al. Tumor suppression in the absence of p53-mediated cell cycle arrest, apoptosis, and senescence. Cell. 2012;149(6):1269–83. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvesen GS. Caspases: opening the boxes and interpreting the arrows. Cell Death Differ. 2002;9(1):3–5. doi: 10.1038/sj.cdd.4400963. [DOI] [PubMed] [Google Scholar]
- 37.Brogliato AR, Moor AN, Kesl SL, Guilherme RF, Georgii JL, Peters-Golden M, et al. Critical role of 5-lipoxygenase and heme oxygenase-1 in wound healing. J Invest Dermatol. 2014;134(5):1436–45. doi: 10.1038/jid.2013.493. [DOI] [PubMed] [Google Scholar]
- 38.Homayouni Moghadam F, Dehghan M, Zarepur E, Dehlavi R, Ghaseminia F, Ehsani S, et al. Oleo gum resin of Ferula assa-foetida L. ameliorates peripheral neuropathy in mice. J Ethnopharmacol. 2014;154(1):183–9. doi: 10.1016/j.jep.2014.03.069. [DOI] [PubMed] [Google Scholar]
- 39.Chen JH, Stoeber K, Kingsbury S, Ozanne SE, Williams GH, Hales CN. Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts. J Biol Chem. 2004;279(47):49439–46. doi: 10.1074/jbc.M409153200. [DOI] [PubMed] [Google Scholar]
- 40.Xing Y, Li N, Zhou D, Chen G, Jiao K, Wang W, et al. Sesquiterpene coumarins from ferula sinkiangensis act as neuroinflammation inhibitors. Planta Med. 2017;83(1–02):135–42. doi: 10.1055/s-0042-109271. [DOI] [PubMed] [Google Scholar]
- 41.Asghari J, Atabaki V, Baher E, Mazaheritehrani M. Identification of sesquiterpene coumarins of oleo-gum resin of Ferula assa-foetida L. from the Yasuj region. Nat Prod Res. 2016;30(3):350–3. doi: 10.1080/14786419.2015.1050669. [DOI] [PubMed] [Google Scholar]
- 42.Znati M, Filali I, Jabrane A, Casanova J, Bouajila J, Ben Jannet H. Chemical composition and in vitro evaluation of antimicrobial, antioxidant and antigerminative properties of the seed oil from the tunisian endemic ferula tunetana pomel ex batt. Chem Biodivers. 2017;14(1) doi: 10.1002/cbdv.201600116. [DOI] [PubMed] [Google Scholar]
- 43.Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–96. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 44.Wang LY, Tang ZJ, Han YZ. Neuroprotective effects of caffeic acid phenethyl ester against sevofluraneinduced neuronal degeneration in the hippocampus of neonatal rats involve MAPK and PI3K/Akt signaling pathways. Mol Med Rep. 2016;14(4):3403–12. doi: 10.3892/mmr.2016.5586. [DOI] [PubMed] [Google Scholar]
- 45.Tempark T, Chatproedprai S, Wananukul S. Localized contact dermatitis from Ferula assa-foetida oleo-gum-resin essential oil, a traditional topical preparation for stomach ache and flatulence. Indian J Dermatol Venereol Leprol. 2016;82(4):467. doi: 10.4103/0378-6323.182969. [DOI] [PubMed] [Google Scholar]
- 46.Hadavand Mirzaei H, Hasanloo T. Assessment of chemical composition of essential oil of Ferula assa-foetida oleo-gum-resin from two different sites of Yazd province in center of Iran. Research Journal of Pharmacognosy. 2014;1(2):51–4. [Google Scholar]
- 47.Li RW, David Lin G, Myers SP, Leach DN. Anti-inflammatory activity of Chinese medicinal vine plants. J Ethnopharmacol. 2003;85(1):61–7. doi: 10.1016/S0378-8741(02)00339-2. [DOI] [PubMed] [Google Scholar]