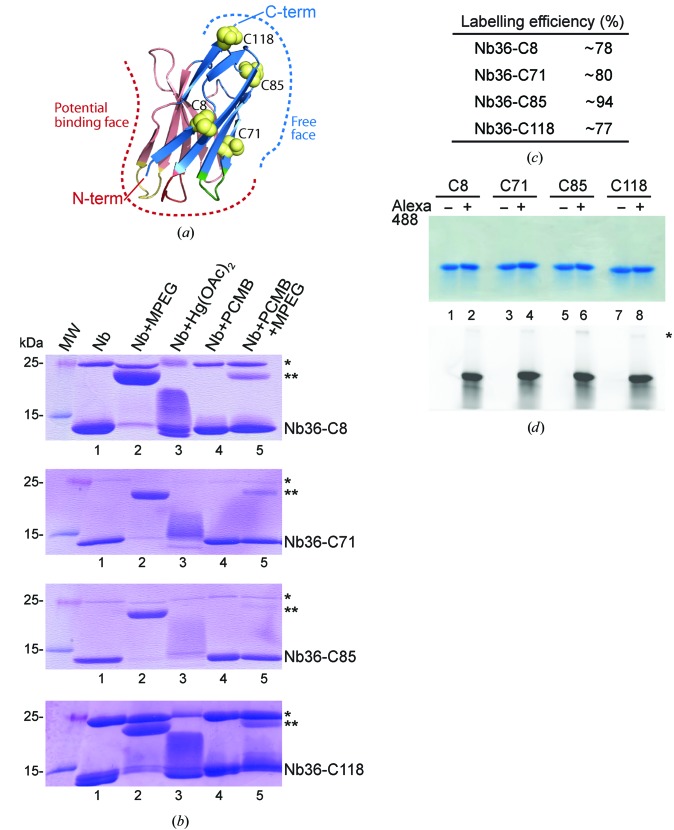
Figure 1.
Site-specific labelling of Nbs. (a) Overview of a Nb structure (exemplified here by PDB entry 3p0g; Rasmussen et al., 2011 ▸) with the face in red observed to contribute to antigen binding (potential binding face); the face in blue has never been observed to directly interact during antigen binding (free face). The positions of the four Ser-to-Cys mutations are indicated as yellow spheres on the free face of the Nb. (b) Nonreduced SDS–PAGE of the labelling reactions with the dimeric form of Nb36 (*) and MPEG-labelled Nb36 (**) indicated. (c) Quantification of labelling efficiency based on the MPEG–maleimide assay. (d) Alexa Fluor 488 labelling of the four cysteine-mutated Nbs. Nonreduced SDS–PAGE analysis of labelled and unlabelled Nbs (top) and the corresponding fluorescence scan of the gel (bottom). * indicates the fraction of dimeric Nb36 nonspecifically labelled with Alexa Fluor 488.