Abstract
Background
The prevalence of cerebral microbleeds (CMBs) in gradient echo T2*‐weighted brain MRI has a positive correlation with hemorrhagic stroke incidence. However, the prevalence of CMBs in patients with left ventricular assist devices (LVADs) has not been evaluated. We evaluated the prevalence of CMBs and the relationship with hemorrhagic stroke incidence in patients with LVADs.
Method and Results
We analyzed results from brain MRI in prospective examinations of 35 consecutive patients who had undergone LVAD explantation for heart transplantation or recovery since 2011. The number and distribution of CMBs were counted, then the relationship between baseline characteristics and adverse events during LVAD support were analyzed. The mean age was 37.7±12.4 years and the mean LVAD duration was 2.43±1.08 years. Thirty‐four (97%) patients had at least one CMB. Nine (26%) developed hemorrhagic stroke during LVAD support, and patients with hemorrhagic stroke had a significantly greater number of CMBs compared with patients without hemorrhagic stroke (5 [interquartile range (IQR), 4–7] versus 9 [IQR, 5–23]; odds ratio 1.14 [95% Confidence Interval (CI), 1.02–1.32], P=0.05). There was no significant relationship between age, LVAD support duration, or systolic blood pressure during LVAD. However, patients who had at least one episode of bacteremia (9 [IQR, 4–16] versus 5 [IQR, 3–7], P=0.06) and pump pocket infection (14 [IQR, 4–27] versus 5 [IQR, 3–7], P=0.08) showed a trend toward a greater number of CMBs than patients without bacteremia.
Conclusions
Thirty‐four (97%) patients with continuous‐flow LVAD had at least one CMB, and the number of CMBs were more prevalent in patients with hemorrhagic stroke and in patients with LVAD‐related infection.
Keywords: cerebral microbleeds, hemorrhagic, MRI, stroke, ventricular assist device
Subject Categories: Intracranial Hemorrhage, Cardiomyopathy, Cardiovascular Surgery, Magnetic Resonance Imaging (MRI)
Clinical Perspective
What Is New?
This study evaluated, for the first time, the prevalence of cerebral microbleeds using MRI in patients with continuous‐flow left ventricular assist devices.
Cerebral microbleeds have been found to have a positive correlation with hemorrhagic stroke incidence in the general population.
We found that nearly all patients with continuous‐flow left ventricular assist devices had at least one cerebral microbleed lesion.
There was also a positive correlation with hemorrhagic stroke in this sample, particularly in patients with continuous‐flow left ventricular assist device–related infection.
What Are the Clinical Implications?
The high prevalence of cerebral microbleeds gives us further insight about risk factors for hemorrhagic stroke in patients with continuous‐flow left ventricular assist devices and the importance of identifying preventive strategies.
Introduction
Implantable continuous‐flow left ventricular assist devices (LVADs) have become prevalent for patients with end‐stage heart failure.1 Although a continuous‐flow LVAD provides better clinical results compared with older pulsatile LVADs because of lower LVAD‐related complications including stroke rate, stroke remains a major adverse event in these patients.2, 3, 4 Several previous studies have reported that the incidence of stroke in patients with continuous‐flow LVAD have remained between 0.06 and 0.16 events per patient‐year.4, 5, 6, 7 Notably, hemorrhagic stroke can deteriorate neurological function to a greater degree than ischemic stroke.4 There are several possible causes of hemorrhagic stroke in patients with LVAD, such as anticoagulation, hemorrhagic conversion after infarction, the acquired von Willebrand syndrome as a result of mechanical destruction and proteolysis of high‐molecular‐weight multimers of von Willebrand factor,8 and rupture of histologically fragile vessels caused by nonphysiological continuous pulseless flow. However, studies that have evaluated risk factors for hemorrhagic stroke in patients with LVADs are limited.5
It has been reported that cerebral microbleeds (CMBs) shown by brain MRI are predictive of symptomatic cerebral hemorrhage in patients with various causes.9, 10 In general population studies, it has been reported that several factors (eg, male sex, older age, elevated blood pressure [BP]) show positive correlations with the number of CMBs,11, 12, 13 while correlations with aspirin and warfarin administration, which are mandatory for patients with LVAD, remain controversial.14, 15, 16, 17 Unfortunately, the prevalence of CMBs in patients receiving LVAD support has not been evaluated, because they are unable to undergo an MRI examination. In the present study, we evaluated the prevalence of CMBs and their relationship with occurrence of hemorrhagic stroke in patients with LVADs after LVAD explantation.
Methods
Patients
The ethical committee of our hospital approved this study. The patient cohort of the present study is detailed in Figure 1A. Between January 2006 and May 2016, 122 patients underwent continuous‐flow LVAD implantation for end‐stage heart failure at our institution. Of those, 50 patients underwent LVAD explantation (45 for heart transplantation, 5 for native heart recovery), of whom 4 underwent heart transplantation before 2011, when we instituted prospective brain MRI examinations after LVAD explantation. Of the remaining 46 patients, 11 could not undergo brain MRI because of death during the acute phase after heart transplantation (n=1), the presence of another implanted mechanical device (eg, implantable cardioverter‐defibrillator) (n=4), young age (n=2), requirement of central extracorporeal membrane oxygenation support for primary graft failure (n=2), requirement of prolonged chemotherapy for post‐transplant lymphoproliferative disease (n=1), and refusal to undergo an MRI examination (n=1). Thus, we performed prospective brain MRI examinations in 35 patients after undergoing LVAD explantation. The purpose of brain MRI examinations was explained to and informed consent was provided by the 35 patients.
Figure 1.
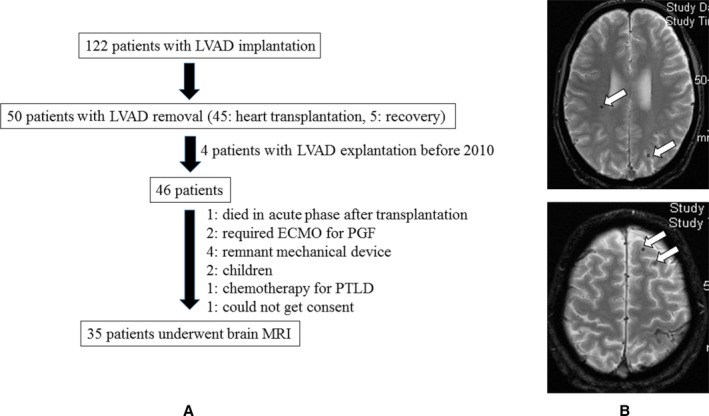
A, Schema of patient selection and purpose of brain MRI examination or no such examination in all 122 patients. B, Cerebral microbleeds were defined as homogeneous round foci <10 mm diameter with low signal intensity shown by T2*‐weighted MRI (white arrow). ECMO indicates extracorporeal membrane oxygenation; LVAD, left ventricular assist device; PGF, primary graft failure; PTLD, post‐transplant lymphoproliferative disorder.
Surgical Procedures
All 35 patients underwent LVAD implantation thorough a median sternotomy with a systemic cardiopulmonary bypass, during which the inflow cannula was inserted into the apex of the left ventricle and the outflow graft was anastomosed to the ascending aorta. Heart transplantation surgery was performed thorough a re‐median sternotomy with a bicaval or modified bicaval technique. The procedure used for patients with native heart recovery has been reported.18, 19
Anticoagulant and Medical Therapy During LVAD Support
Following LVAD implantation, all patients received a standardized heart failure prevention medical regimen, including neurohormonal antagonists, diuretics, and antiarrhythmic agents, as needed. Antiplatelet therapy with aspirin (100 mg daily) was also implemented on the next day of LVAD implantation and maintained throughout LVAD support unless any bleeding events occurred. Anticoagulation therapy was also immediately implemented after LVAD implantation and maintained throughout LVAD support at the target international normalized ratio of 2.0 to 2.5 for patients with a HeartMateII (Thoratec) or DuraHeart (Terumo Heart) device, and 2.5 to 3.0 for those with a HeartWare (HeartWare), EVAHEART (SunMedical), or Jarvik 2000 (Jarvik Heart) device. In our hospital, patients routinely visit the hospital and check their international normalized ratio every month. If patients have below or above therapeutic ranges, their international normalized ratio is checked every 2 or 3 days with CoaguCheck (Roche) at home until therapeutic the range is stably achieved.
Definition and Management of LVAD‐Related Adverse Events
A stroke event was defined as a neurological symptom lasting >24 hours with a compatible new lesion shown in brain computed tomography findings, then subdivided into ischemic and hemorrhagic stroke cases. Hemorrhagic stroke included cerebral, cerebellum, and subarachnoid hemorrhage. Subdural hematoma and hemorrhagic infarction with any hemorrhagic transformation was not included as hemorrhagic stroke in the present study. Upon occurrence of an LVAD‐related stroke, the patient was hospitalized and treatment was managed based on discussion among experienced cardiologists, cardiovascular surgeons, and neurologists. The termination and reinitiation of antiplatelet and/or anticoagulation therapy was also managed by the discussion. The indication of selective angiography and treatment of intracranial aneurysm is also discussed with neurosurgeons. Consecutive head computed tomography scans are routinely performed before and after reinitiation of anticoagulant therapy. This multidisciplinary approach is continued until stabilization of symptoms and reinitiated anticoagulant therapy.
A major bleeding event was defined as an episode of internal or external bleeding that required any type of procedure or necessitated transfusion of packed red blood cells (2 U) within 24 hours of the event. In the present study, major bleeding events did not include hemorrhagic stroke. At our institution, when a patient with LVAD develops a high fever, we routinely examine blood cultures and investigate the source of infection using computed tomography scan or gallium‐scintigraphy results. An episode of systemic bacteremia was defined as any episode during LVAD support that led to positive blood culture findings. A pump pocket infection was defined as an infection accompanied by an abscess around the LVAD pump. In the present cohort, no patient had any vegetation or abscess in the explanted LVAD pump at the time of heart transplantation or LVAD removal surgery. Heart failure during LVAD support includes both left‐sided heart failure caused by aortic valve insufficiency or device malfunction and right‐sided heart failure. Arrhythmia was defined as ventricular arrhythmia that required cardioconversion or induction of antiarrhythmogenic agents. Pump thrombosis was defined as an evident thrombotic formation accompanied by elevated serum lactate dihydrogen that required pump exchange or increased anticoagulation therapy.
MRI Procedure
Brain MRI examinations were mainly conducted during hospitalization following LVAD explantation performed for heart transplantation or recovery, regardless of the presence of neurological symptoms. The mean period between LVAD explantation (heart transplantation or removal for native heart recovery) and MRI examination was 50±41 days (mean, 39; range 29–51 days). Gradient echo T2*‐weighted MRI was performed with a 3.0‐Tesla system (repetition time/echo time 617/20 ms, flip angle 25°, matrix 256×256) using diffusion‐, T1‐, T2‐, and T2 fluid–attenuated inversion recovery‐weighted sequences with a 5‐mm slice thickness and 1‐mm slice gap. The obtained images were independently assessed by 2 stroke neurologists (S.O. and S.M.) who were blinded to patient characteristics. CMBs were defined as homogeneous round foci <10 mm in diameter with a low signal intensity in T2*‐weighted MRI findings (Figure 1B). The number and distribution of CMBs were recorded using the Brain Observer MicroBleed Scale.20 Differences in assessment were resolved by consensus.
Data Collection
All patient data were obtained from electronic medical records, including baseline characteristics, comorbidities, and type and duration of LVAD. Major adverse events requiring readmission during LVAD support were also recorded, which included cerebral events, symptomatic heart failure, major bleeding events, cardiac arrhythmia, and LVAD‐related and ‐unrelated infections. Systolic BP during LVAD was obtained just before undergoing heart transplantation or LVAD explantation surgery.
Statistical Analysis
Quantitative variables are presented as means with SDs or medians with interquartile ranges (IQRs), and were compared using the Welch t test. Qualitative variables are presented as frequencies with percentages, and were compared using the Fisher t test. Correlation between variables was assessed with the use of Pearson's correlation coefficient. The odds ratios with 95% confidence intervals (CIs) of risk factors associated with hemorrhagic stroke were estimated with the use of the logistic regression model with Firth's penalized likelihood. This can provide a solution to the phenomenon of monotone likelihood that causes parameter estimates of the usual logistic regression model to diverge, with infinite SEs (in fact, such a phenomenon existed in some factors, eg, ischemic cardiomyopathy, HeartMate II, Jarvik 2000, coronary artery disease, and bleeding events). All P values were 2‐sided, and values of P<0.05 were considered to indicate statistical significance. Interobserver reliability was assessed by intraclass correlation coefficient calculation. Statistical analyses were performed using JMP software version 11 (SAS Institute Inc) except when the logistic regression analysis with Firth's penalized likelihood was performed using SAS 9.4 (SAS Institute Inc).
Results
Of 35 patients who underwent brain MRI following LVAD explantation, 9 (26%) developed hemorrhagic stroke during LVAD support. The detail of these 9 hemorrhagic stroke cases is shown in Table S1. Subarachnoid hemorrhage occurred in 4 patients, while cerebral or cerebellum hemorrhage occurred in 6 patients. One patient had separate subarachnoid and cerebellum hemorrhage events. One patient developed subarachnoid hemorrhage 5 days after LVAD implantation, whereas the other 8 patients developed hemorrhagic stroke in the chronic period at a median of 477 days after LVAD implantation. In the 8 patients with hemorrhagic stroke in the chronic period, 3 patients had ongoing pump pocket infection that was being treated by debridement and antibiotics, 1 patient had latent pump pocket infection that was first noticed at the time of heart transplantation, 1 patient had driveline infection that was treated at home, 1 patient had no signs of infection but had an episode of bacteremia 1 year before stroke, and 2 patients had no signs of infection nor an episode of bacteremia. One patient developed transient ataxia and 3 patients developed transient disturbance of consciousness. None of these 9 patients underwent selective angiography after hemorrhagic stroke because of a relatively small lesion without serious neurological dysfunction. All patients fully recovered without neurological deficit at the time of heart transplantation.
All baseline characteristics, systolic BP during LVAD, LVAD support duration, and adverse events during LVAD support of the 35 patients are shown in Table 1 and compared between patients with and without hemorrhagic stroke. This cohort included only patients with bridge to transplant, thus the mean age was 37.7±12.4 years, and patients with hemorrhagic stroke during LVAD support showed a trend toward younger age as compared with patients without hemorrhagic stroke. Twenty‐seven (77%) of these patients were men and 25 (71%) had idiopathic dilated cardiomyopathy. A DuraHeart device was used as the implanted device in 19 patients (54%), a HeartMate II device in 5 patients (14%), and a HeartWare device in 5 patients (14%). As for comorbidities, 5 (14%) patients had diabetes mellitus, none of whom had a stroke before LVAD implantation. There were no differences in pathogenesis, presence of diabetes mellitus, pump type, and systolic BP during LVAD support between patients with and without hemorrhagic stroke. These 35 patients had a mean of 2.43 years of support with an LVAD. During LVAD support, 13 patients had at least one case of bacteremia, with the microorganisms as follows: methicillin‐sensitive Staphylococcus epidermidis (3), methicillin‐sensitive Staphylococcus aureus (2), Corynebacterium (2), methicillin‐resistant S aureus (1), other Staphylococcus (2), Enterococcus (1), Bacillus species (1), and Campylobacter (1). Interestingly, patients with hemorrhagic stroke showed significantly higher rates of bacteremia, pump pocket infection, and heart failure incidence.
Table 1.
Patient Characteristics at Baseline and During LVAD and Adverse Events During LVAD
| Overall | Hemorrhagic Stroke (−) | Hemorrhagic Stroke (+) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|---|
| N=35 | n=26 | n=9 | |||
| Before LVAD implantation | |||||
| Age, y | 37.7±12.4 | 40.2±11.2 | 30.7±13.5 | 0.93 (0.85–1.00) | 0.08 |
| Men, No. (%) | 27 (77) | 21 (81) | 6 (67) | 0.48 (0.10–2.53) | 0.39 |
| Body surface area, m2 | 1.59±0.16 | 1.59±0.15 | 1.57±0.17 | 0.37 (0.00–66.9) | 0.71 |
| Body mass index, kg/m2 | 19.4±2.4 | 19.7±2.3 | 18.7±2.7 | 0.86 (0.58–1.19) | 0.41 |
| Pathogenesis | |||||
| Idiopathic dilated cardiomyopathy, No. (%) | 25 (71) | 19 (73) | 6 (67) | 0.71 (0.14–3.57) | 0.68 |
| Ischemic cardiomyopathy, No. (%) | 4 (11) | 4 (15) | 0 (0) | 0.26 (0.01–7.55) | 0.44 |
| Other, No. (%) | 6 (17) | 4 (15) | 2 (22) | 1.67 (0.26–10.8) | 0.59 |
| Devices | |||||
| Axial flow | 8 (23) | 6 (23) | 2 (22) | 1.05 (0.18–61.5) | 0.96 |
| Centrifugal flow | 27 (77) | 20 (77) | 7 (78) | 0.96 | |
| DuraHeart, No. (%) | 19 (54) | 14 (54) | 5 (56) | 1.05 (0.24–4.72) | 0.95 |
| HeartMate II, No. (%) | 5 (14) | 5 (19) | 0 (0) | 0.21 (0.01–5.39) | 0.34 |
| HeartWare, No. (%) | 5 (14) | 4 (15) | 1 (11) | 0.88 (0.10–7.79) | 0.91 |
| EVAHEART, No. (%) | 3 (9) | 2 (8) | 1 (11) | 1.73 (0.15–20.4) | 0.66 |
| Jarvik 2000, No. (%) | 3 (9) | 1 (4) | 2 (22) | 5.57 (0.48–67.6) | 0.17 |
| Comorbidities before LVAD implantation | |||||
| Diabetes mellitus, No. (%) | 5 (14) | 4 (15) | 1 (11) | 0.88 (0.10–7.79) | 0.91 |
| Coronary artery disease, No. (%) | 4 (11) | 4 (15) | 0 (0) | 0.26 (0.01–7.55) | 0.44 |
| Stroke, No. (%) | 0 (0) | 0 (0) | 0 (0) | ||
| LVDd, mm | 70.8±12.6 | 70.8±12.1 | 64.2±9.9 | 0.96 (0.88–1.04) | 0.31 |
| LVDs, mm | 65.0±13.0 | 65.0±12.6 | 58.2±9.8 | 0.96 (0.88–1.04) | 0.32 |
| LVEF, % | 21.2±7.6 | 21.6±8.1 | 20.6±5.9 | 0.99 (0.87–1.12) | 0.86 |
| Pre‐LVAD laboratory data | |||||
| Hemoglobin, g/dL | 11.0±1.8 | 11.0±1.9 | 11.3±1.5 | 1.08 (0.69–1.74) | 0.71 |
| Serum creatinine, mg/dL | 1.16±0.54 | 1.18±0.55 | 1.12±0.57 | 0.87 (0.20–3.77) | 0.85 |
| Serum total bilirubin, mg/dL | 1.49±1.45 | 1.27±1.17 | 2.20±2.06 | 1.42 (0.86–2.34) | 0.17 |
| Serum albumin, mg/dL | 3.58±0.59 | 3.63±0.61 | 3.44±0.53 | 0.59 (0.16–2.14) | 0.44 |
| Systolic blood pressure during LVAD, mm Hg | 79.2±10.4 | 80.1±10.2 | 76.3±11.3 | 0.97 (0.88–1.06) | 0.51 |
| LVAD support duration, y | 2.43±1.08 | 2.27±1.04 | 2.91±1.07 | 1.79 (0.78–4.11) | 0.17 |
| Adverse events during LVAD support | |||||
| Bacteremia, No. (%) | 13 (37) | 6 (23) | 7 (78) | 9.45 (1.68–53.3) | 0.01 |
| Pump pocket infection, No. (%) | 6 (17) | 1 (4) | 5 (56) | 20.8 (2.3–187.6) | 0.01 |
| Driveline infection, No. (%) | 13 (37) | 9 (35) | 4 (44) | 1.51 (0.33–6.90) | 0.60 |
| Bleeding events, No. (%) | 3 (9) | 3 (12) | 0 (0) | 0.35 (0.11–11.8) | 0.56 |
| Heart failure, No. (%) | 10 (29) | 4 (15) | 6 (67) | 9.29 (1.69–51.1) | 0.01 |
| Arrhythmia, No. (%) | 3 (9) | 2 (8) | 1 (11) | 1.73 (0.15–20.4) | 0.66 |
| Pump thrombosis, No. (%) | 4 (11) | 2 (8) | 2 (22) | 3.27 (0.39–27.5) | 0.28 |
| Ischemic stroke, No. (%) | 4 (11) | 3 (12) | 1 (11) | 1.19 (0.12–11.6) | 0.89 |
CI indicates confidence interval; LVAD, left ventricular assist device; LVDd, left ventricular end‐diastolic dimension; LVDs, left ventricular end‐systolic dimension; LVEF, left ventricular ejection fraction.
We analyzed the relationship between the number of CMBs and hemorrhagic stroke in the 35 patients (Table 2). Interobserver agreement for CMB count was excellent, with an intraclass correlation coefficient value of 0.91. Both neurologists detected at least one CMB site in 31 (89%) patients, while there was only one (3%) patient without CMBs according to the evaluation of both neurologists. Patients who experienced a hemorrhagic stroke during LVAD support showed a trend toward the presence of a greater number of CMBs compared with patients without hemorrhagic stroke 9 (IQR: 5‐23) vs 5 (IQR 4‐7), P=0.09), although it did not reach statistical significance because of the limited number of patients. However, the number of all CMBs had a positive relationship with hemorrhagic stroke (OR, 1.14; 95% CI, 1.02–1.32 [P=0.05]). There was a trend that the number of lobar CMBs (OR, 1.13; 95% CI, 0.97–1.31 [P=0.13]) and deep CMBs (OR, 1.65; 95% CI, 0.94–2.99 [P=0.08]) had a positive relationship with hemorrhagic stroke. The prevalence of silent hemorrhage >10 mm in diameter was prevalent in patients with hemorrhagic stroke (44% versus 12% [P=0.05]; OR, 5.50; 95% CI, 0.94–32.2 [P=0.06]).
Table 2.
MRI Findings of Patients With and Without Hemorrhagic Stroke During LVAD Support
| Hemorrhagic Stroke (−) n=26 | Hemorrhagic Stroke (+) n=9 | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| No. of CMBs | ||||
| All CMBs | 5 (4–7) | 9 (5–23) | 1.14 (1.02–1.32) | 0.05 |
| Lobar CMBs | 4 (2–6) | 4 (0–16) | 1.13 (0.97–1.31) | 0.13 |
| Deep CMBs | 1 (0–1) | 2 (0–4) | 1.65 (0.94–2.91) | 0.08 |
| Infra CMBs | 1 (0–2) | 1 (0–3) | 1.38 (0.78–2.46) | 0.27 |
| Presence of silent hemorrhage >10 mm, No. (%) | 3 (12) | 4 (44) | 5.50 (0.94–32.2) | 0.06 |
CMBs indicates cerebral microbleeds; LVAD, left ventricular assist device.
We also analyzed the relationship of number of CMBs with baseline characteristics and adverse events during LVAD support (Table 3; Figures 2 and 3). There was no significant linear correlation between CMB count and age, systolic BP during LVAD, and LVAD support duration (Figure 2A through 2C). Furthermore, there were no significant differences in sex, etiology, presence of diabetes mellitus, and pump type. Interestingly, 13 patients with an episode of bacteremia showed a trend toward the presence of a greater number of CMBs compared with patients those with none 9 (IQR: 4‐16) vs 5 (IQR: 3‐7), P=0.06) (Figure 3A). In addition, patients who had experienced a pocket infection also showed a trend toward the presence of a greater number of CMBs. (14 [4–27] versus 5 [3–7], P=0.08) (Figure 3B). On the other hand, there was no significant difference between patients with and without a driveline infection 4 (IQR:3‐9) vs 5 (IQR:3‐9), P=0.77) (Figure 3C). To exclude the impact of infection during LVAD support, we performed analysis of the subset of patients who had never developed an infection during the period of LVAD support, which revealed no statistical correlation between the number of CMBs and age (r=0.22, P=0.40), systolic BP (r=0.12, P=0.68), or LVAD support duration (r=0.06, P=0.84).
Table 3.
CMBs According to Baseline Characteristics and Adverse Events During LVAD
| No. of CMBs | P Value | ||
|---|---|---|---|
| Men (n=27) | Women (n=8) | ||
| Sex | 5 (3–9) | 6 (4–9) | 0.38 |
| ICM (n=4) | Non‐ICM (n=31) | ||
| ICM | 9 (3–12) | 5 (4–9) | 0.94 |
| DM (+) (n=5) | DM (−) (n=30) | ||
| DM | 5 (4–8) | 5 (2–9) | 0.31 |
| Axial flow (n=8) | Centrifugal flow (n=27) | ||
| Pump type | 4 (1–5) | 6 (4–9) | 0.14 |
| Complication during LVAD support | |||
| Bacteremia (+) (n=13) | Bacteremia (−) (n=22) | ||
| Bacteremia | 9 (4–16) | 5 (3–7) | 0.06 |
| Infection (+) (n=6) | Infection (−) (n=29) | ||
| Pocket infection | 14 (4–27) | 5 (3–7) | 0.08 |
| Infection (+) (n=13) | Infection (−) (n=22) | ||
| Driveline infection | 4 (3–9) | 5 (3–9) | 0.77 |
| Heart failure (+) (n=10) | Heart failure (−) (n=25) | ||
| Heart failure | 5 (4–11) | 6 (2–9) | 0.40 |
| Arrhythmia (+) (n=3) | Arrhythmia (−) (n=32) | ||
| Arrhythmia | 9 (3–9) | 5 (3–9) | 0.81 |
| Bleeding (+) (n=4) | Bleeding (−) (n=31) | ||
| Bleeding | 7 (6–14) | 5 (3–9) | 0.55 |
| Thrombosis (+) (n=4) | Thrombosis (−) (n=31) | ||
| LVAD thrombosis | 4 (1–8) | 6 (4–9) | 0.21 |
| Ischemic stroke (+) (n=4) | Ischemic stroke (−) (n=31) | ||
| Ischemic stroke | 5 (1–9) | 5 (4–9) | 0.25 |
CMBs indicates cerebral microbleeds; DM, diabetes mellitus; ICM, ischemic cardiomyopathy; LVAD, left ventricular assist device.
Figure 2.
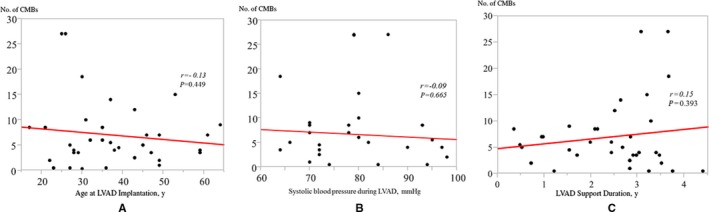
Relationship of the number of cerebral microbleeds (CMBs) with (A) age, (B) systolic blood pressure during left ventricular assist device (LVAD), and (C) LVAD support duration in patients without any episode of bacteremia or pump pocket infection.
Figure 3.

Number of cerebral microbleeds (CMBs) in patients with and without infectious complications during left ventricular assist device (LVAD) support. A, bacteremia, (B) pump pocket infection, and (C) driveline exit site infection. Red indicates patients with hemorrhagic stroke; black, patients without hemorrhagic stroke).
Discussion
The primary findings of the present study are as follows: (1) patients who experienced symptomatic hemorrhagic stroke during LVAD support had a high rate of bacteremia or LVAD pump pocket infection; (2) the MRI findings showed that the majority of patients with an LVAD had at least one CMB regardless of LVAD‐related infection, while those with hemorrhagic stroke had a greater number of infections compared with those without hemorrhagic stroke; (3) the number of CMBs did not have a relationship with age, systolic BP, LVAD support duration, or baseline characteristics, whereas relationships were likely seen with bacteremia and LVAD pump pocket infection.
In the present study, 26% patients had an episode of hemorrhagic stroke. The incidence of hemorrhagic stroke was higher than in previous reports.21, 22 Our cohort included only patients who successfully bridged to heart transplantation or LVAD removal for native heart recovery, therefore the incidence of hemorrhagic stroke might be higher if all of our patients with LVAD are evaluated. One of the reasons for this discrepancy might be explained by racial differences. Most previous reports that evaluated risk factors for stroke in patients with LVAD were comprised of only white and/or black patients. In addition, there has been only one study that has compared the incidence of stroke between white and black patients with LVAD,23 and a study with Asian patients has never been reported. The incidence of cerebrovascular accidents in the general population is different depending on racial differences.24, 25 Therefore, it is possible that the prevalence of stroke would differ among several races. Further studies should be performed to evaluate the risk factors and causes of stroke in elderly patients in Asian countries.
It is widely known that elevated BP and older age are important risk factors for hemorrhagic stroke in the general population,26 while it has been reported that several factors (eg, ischemic cause, male sex, older age) showed positive correlations with the number of CMBs in general populations.11, 12, 13 Previous studies have reported that the prevalence of CMBs ranged from 3.1% to 23.5% in healthy persons without cerebrovascular disease, 18% to 68% in patients with ischemic stroke, and up to 47% to 80% in patients with intracranial hemorrhage.12, 27, 28 To the best of our knowledge, this is the first study to investigate the prevalence of CMBs in patients with LVAD. Our cohort was comprised of young patients with a low prevalence of ischemic pathogenesis because of epidemiological differences in coronary artery disease.29 None of our patients had a stroke before LVAD implantation and they were relatively young. In addition, nearly all patients had low BP before receiving the LVAD because of heart failure; therefore, we speculate that the possibility of existing CMBs before implantation was low. Nevertheless, almost all patients had at least one CMB. It has been shown that CMBs are caused by fractures of fragile small vessels in the brain.30 These results may indicate that patients with LVADs are vulnerable to intracranial bleeding, even in young patients with low BP, and symptomatic hemorrhagic stroke in patients with LVADs caused by spontaneous bleeding from fragile small vessels. Tabit et al31 concluded that continuous flow leads to altered angiogenesis and is associated with higher nonsurgical bleeding via an elevated level of angiopoietin‐2. While abnormal vascular formation might cause bleeding in the brain, further evaluations of the mechanism of vascular fragility in the brain of patients with an LVAD are warranted.
It is particularly important that occurrence of an LVAD‐related infection (bacteremia and/or LVAD pump pocket infection, not driveline exit site infection) might have positive relationships with symptomatic hemorrhagic stroke and the number of CMBs. These results are in accordance with previous reports showing that persistent bloodstream infection increases the risk of hemorrhagic stroke in patients with LVADs.32, 33 Furthermore, it has been noted that infectious diseases that accompany bloodstream infection (eg, infective endocarditis) frequently cause mycotic angiopathy and hemorrhagic cerebral complications.34, 35, 36, 37 The prevalence of CMBs in patients with endocarditis have been reported to be 50% to 60%.35, 38 In addition, Masuda et al36 reported autopsy findings showing that pyogenic arteritis without an obvious mycotic aneurysm was often present in the intracranial vessels of patients with infective endocarditis who died of hemorrhagic stroke. An LVAD‐related infection such as pump pocket infection can increase the risk of prolonged bacteremia. It is also reasonable to speculate that pyogenic arteritis, anticoagulation, or continuous flow–induced vascular fragility can accelerate intracranial bleeding. We found that a driveline infection without the presence of bacteremia was not a risk factor for hemorrhagic stroke or increase in the number of CMBs. Therefore, precaution of LVAD pump pocket infection and systemic bacteremia and early aggressive treatment of a driveline infection should be of highest priority to prevent hemorrhagic stroke in patients with LVADs.
Study Limitations
The present study has several limitations. First, even though we prospectively performed brain MRI examinations in a relatively homogenous cohort, this is a single‐center study and the number of patients who underwent those examinations was only 35, possibly resulting in insufficient statistical power. In addition, the mean age of our patients was 37.7 years and different from the cohorts of other previous LVAD studies. Although anticoagulation level with warfarin was strictly managed, it was impossible to calculate the duration of a subtherapeutic or supratherapeutic range of anticoagulation level in each patient. Second, patients who died during LVAD support did not receive an MRI examination and were not enrolled. Patients who died of stroke might have more specific MRI findings and the results could be different. Third, although most previous studies on CMBs used 1‐ to 1.5‐Tesla MRI, the present study used a 3.0‐Tesla system. Therefore, the possibility of overestimation of CMBs cannot be eliminated. In addition, it was impossible to distinguish completely from primary cerebral hemorrhage from hemorrhagic transformation of cerebral infarction, particularly if the hemorrhagic lesion was large. Finally, although there was no statistical difference between axial flow and use of a centrifugal pump, our cohort was supported by several different types of LVADs. Thus, we cannot completely eliminate the possibility that CMBs developed before LVAD implantation or during LVAD implantation surgery, heart transplantation surgery, or explantation surgery that required cardiopulmonary bypass.
Conclusions
A total of 97% of our patients supported by a continuous‐flow LVAD had evidence of CMBs on postexplantation MRI, and the CMB number showed a positive correlation with a hemorrhagic stroke episode. The occurrence of hemorrhagic stroke in patients with a continuous‐flow LVAD may be related to vascular fragility, especially in patients with LVAD‐related infection. To reduce the incidence of hemorrhagic stroke, additional investigations regarding its mechanism are warranted.
Disclosures
None.
Supporting information
Table S1. Patients With Hemorrhagic Stroke During LVAD Support
(J Am Heart Assoc. 2017;6:e005955 DOI: 10.1161/JAHA.117.005955.)28893764
References
- 1. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–1504. [DOI] [PubMed] [Google Scholar]
- 2. Kato TS, Ota T, Schulze PC, Farr M, Jorde U, Takayama H, Naka Y, Yamashita T, Mancini DM. Asymmetric pattern of cerebrovascular lesions in patients after left ventricular assist device implantation. Stroke. 2012;43:872–874. [DOI] [PubMed] [Google Scholar]
- 3. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM III, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous‐flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. [DOI] [PubMed] [Google Scholar]
- 4. Willey JZ, Gavalas MV, Trinh PN, Yuzefpolskaya M, Reshad Garan A, Levin AP, Takeda K, Takayama H, Fried J, Naka Y, Topkara VK, Colombo PC. Outcomes after stroke complicating left ventricular assist device. J Heart Lung Transplant. 2016;35:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyle AJ, Jorde UP, Sun B, Park SJ, Milano CA, Frazier OH, Sundareswaran KS, Farrar DJ, Russell SD. Pre‐operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol. 2014;63:880–888. [DOI] [PubMed] [Google Scholar]
- 6. Harvey L, Holley C, Roy SS, Eckman P, Cogswell R, Liao K, John R. Stroke after left ventricular assist device implantation: outcomes in the continuous‐flow era. Ann Thorac Surg. 2015;100:535–541. [DOI] [PubMed] [Google Scholar]
- 7. Kato TS, Schulze PC, Yang J, Chan E, Shahzad K, Takayama H, Uriel N, Jorde U, Farr M, Naka Y, Mancini D. Pre‐operative and post‐operative risk factors associated with neurologic complications in patients with advanced heart failure supported by a left ventricular assist device. J Heart Lung Transplant. 2012;31:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uriel N, Pak SW, Jorde UP, Jude B, Susen S, Vincentelli A, Ennezat PV, Cappleman S, Naka Y, Mancini D. Acquired von Willebrand syndrome after continuous‐flow mechanical device support contributes to a high prevalence of bleeding during long‐term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213. [DOI] [PubMed] [Google Scholar]
- 9. Bokura H, Saika R, Yamaguchi T, Nagai A, Oguro H, Kobayashi S, Yamaguchi S. Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke. 2011;42:1867–1871. [DOI] [PubMed] [Google Scholar]
- 10. Nishikawa T, Ueba T, Kajiwara M, Fujisawa I, Miyamatsu N, Yamashita K. Cerebral microbleeds predict first‐ever symptomatic cerebrovascular events. Clin Neurol Neurosurg. 2009;111:825–828. [DOI] [PubMed] [Google Scholar]
- 11. Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J. Higher ambulatory blood pressure relates to new cerebral microbleeds: 2‐year follow‐up study in lacunar stroke patients. Stroke. 2013;44:978–983. [DOI] [PubMed] [Google Scholar]
- 12. Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology. 2006;66:165–171. [DOI] [PubMed] [Google Scholar]
- 13. Staals J, van Oostenbrugge RJ, Knottnerus IL, Rouhl RP, Henskens LH, Lodder J. Brain microbleeds relate to higher ambulatory blood pressure levels in first‐ever lacunar stroke patients. Stroke. 2009;40:3264–3268. [DOI] [PubMed] [Google Scholar]
- 14. Kim CK, Kwon HT, Kwon HM. No significant association of aspirin use with cerebral microbleeds in the asymptomatic elderly. J Neurol Sci. 2012;319:56–58. [DOI] [PubMed] [Google Scholar]
- 15. Lovelock CE, Cordonnier C, Naka H, Al‐Shahi Salman R, Sudlow CL, Sorimachi T, Werring DJ, Gregoire SM, Imaizumi T, Lee SH, Briley D, Rothwell PM. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke. 2010;41:1222–1228. [DOI] [PubMed] [Google Scholar]
- 16. Orken DN, Kenangil G, Uysal E, Forta H. Cerebral microbleeds in ischemic stroke patients on warfarin treatment. Stroke. 2009;40:3638–3640. [DOI] [PubMed] [Google Scholar]
- 17. Vernooij MW, Haag MD, van der Lugt A, Hofman A, Krestin GP, Stricker BH, Breteler MM. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2009;66:714–720. [DOI] [PubMed] [Google Scholar]
- 18. Kashiyama N, Toda K, Miyagawa S, Nishi H, Yoshikawa Y, Fukushima S, Yoshioka D, Saito T, Sawa Y. Initial experience of EVAHEART explantation after continuous‐flow LVAD off test with percutaneous occlusion balloon. J Artif Organs. 2014;17:366–369. [DOI] [PubMed] [Google Scholar]
- 19. Yoshioka D, Toda K, Sakaguchi T, Miyagawa S, Nishi H, Yoshikawa Y, Fukushima S, Saito S, Saito T, Shibasaki I, Sakata Y, Ohtani T, Sawa Y. Initial report of bridge to recovery in a patient with DuraHeart LVAD. J Artif Organs. 2013;16:386–388. [DOI] [PubMed] [Google Scholar]
- 20. Cordonnier C, Potter GM, Jackson CA, Doubal F, Keir S, Sudlow CL, Wardlaw JM, Al‐Shahi Salman R. Improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke. 2009;40:94–99. [DOI] [PubMed] [Google Scholar]
- 21. Willey JZ, Demmer RT, Takayama H, Colombo PC, Lazar RM. Cerebrovascular disease in the era of left ventricular assist devices with continuous flow: risk factors, diagnosis, and treatment. J Heart Lung Transplant. 2014;33:878–887. [DOI] [PubMed] [Google Scholar]
- 22. Frontera JA, Starling R, Cho SM, Nowacki AS, Uchino K, Hussain MS, Mountis M, Moazami N. Risk factors, mortality, and timing of ischemic and hemorrhagic stroke with left ventricular assist devices. J Heart Lung Transplant. 2017;36:673–683. [DOI] [PubMed] [Google Scholar]
- 23. Meeteren JV, Maltais S, Dunlay SM, Haglund NA, Beth Davis M, Cowger J, Shah P, Aaronson KD, Pagani FD, Stulak JM. A multi‐institutional outcome analysis of patients undergoing left ventricular assist device implantation stratified by sex and race. J Heart Lung Transplant. 2017;36:64–70. [DOI] [PubMed] [Google Scholar]
- 24. Jose PO, Frank AT, Kapphahn KI, Goldstein BA, Eggleston K, Hastings KG, Cullen MR, Palaniappan LP. Cardiovascular disease mortality in Asian Americans. J Am Coll Cardiol. 2014;64:2486–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gunarathne A, Patel JV, Gammon B, Gill PS, Hughes EA, Lip GY. Ischemic stroke in South Asians: a review of the epidemiology, pathophysiology, and ethnicity‐related clinical features. Stroke. 2009;40:e415–e423. [DOI] [PubMed] [Google Scholar]
- 26. Rapsomaniki E, Timmis A, George J, Pujades‐Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al‐Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke. 2006;37:550–555. [DOI] [PubMed] [Google Scholar]
- 29. Lloyd‐Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel‐Smoller S, Wong N, Wylie‐Rosett J, Hong Y. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. [DOI] [PubMed] [Google Scholar]
- 30. Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta‐analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013;44:995–1001. [DOI] [PubMed] [Google Scholar]
- 31. Tabit CE, Chen P, Kim GH, Fedson SE, Sayer G, Coplan MJ, Jeevanandam V, Uriel N, Liao JK. Elevated angiopoietin‐2 level in patients with continuous‐flow left ventricular assist devices leads to altered angiogenesis and is associated with higher nonsurgical bleeding. Circulation. 2016;134:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trachtenberg BH, Cordero‐Reyes AM, Aldeiri M, Alvarez P, Bhimaraj A, Ashrith G, Elias B, Suarez EE, Bruckner B, Loebe M, Harris RL, Zhang JY, Torre‐Amione G, Estep JD. Persistent blood stream infection in patients supported with a continuous‐flow left ventricular assist device is associated with an increased risk of cerebrovascular accidents. J Card Fail. 2015;21:119–125. [DOI] [PubMed] [Google Scholar]
- 33. Aggarwal A, Gupta A, Kumar S, Baumblatt JA, Pauwaa S, Gallagher C, Treitman A, Pappas P, Tatooles A, Bhat G. Are blood stream infections associated with an increased risk of hemorrhagic stroke in patients with a left ventricular assist device? ASAIO J. 2012;58:509–513. [DOI] [PubMed] [Google Scholar]
- 34. Yoshioka D, Toda K, Okazaki S, Sakaguchi T, Miyagawa S, Yoshikawa Y, Sawa Y. Anemia is a risk factor of new intraoperative hemorrhagic stroke during valve surgery for endocarditis. Ann Thorac Surg. 2015;100:16–23. [DOI] [PubMed] [Google Scholar]
- 35. Okazaki S, Sakaguchi M, Hyun B, Nagano K, Tagaya M, Sakata Y, Sakaguchi T, Kitagawa K. Cerebral microbleeds predict impending intracranial hemorrhage in infective endocarditis. Cerebrovasc Dis. 2011;32:483–488. [DOI] [PubMed] [Google Scholar]
- 36. Masuda J, Yutani C, Waki R, Ogata J, Kuriyama Y, Yamaguchi T. Histopathological analysis of the mechanisms of intracranial hemorrhage complicating infective endocarditis. Stroke. 1992;23:843–850. [DOI] [PubMed] [Google Scholar]
- 37. Duval X, Iung B, Klein I, Brochet E, Thabut G, Arnoult F, Lepage L, Laissy JP, Wolff M, Leport C. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med. 2010;152:497–504, W175. [DOI] [PubMed] [Google Scholar]
- 38. Champey J, Pavese P, Bouvaist H, Kastler A, Krainik A, Francois P. Value of brain MRI in infective endocarditis: a narrative literature review. Eur J Clin Microbiol Infect Dis. 2016;35:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patients With Hemorrhagic Stroke During LVAD Support


