Figure 4.
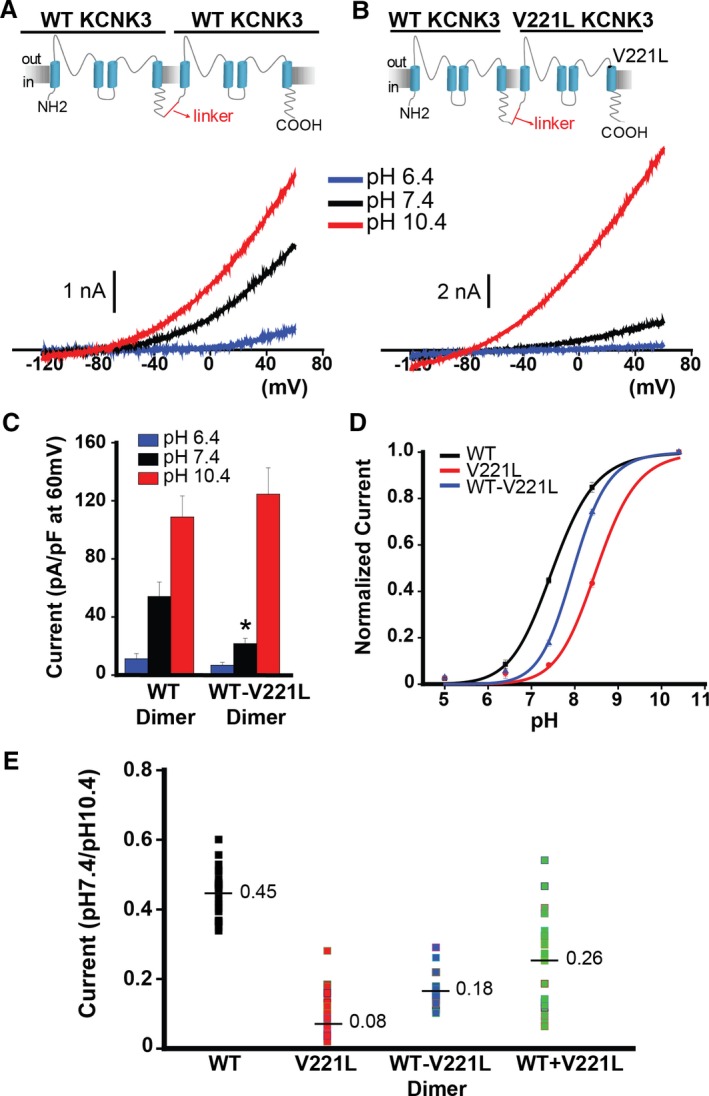
Tandem‐linked KCNK3 heterodimeric channels are functional reporters of KCNK3 heterozygosity. A and B, KCNK3 dimers were engineered by interconnecting 2 KCNK3 subunits with a glycine‐rich linker. The wildtype (WT) KCNK3 homodimer (A) and the WT−V221L KCNK3 heterodimer (B) are depicted, with sample voltage clamp recordings for each condition. Current traces at pH 6.4 (blue), 7.4 (black), and 10.4 (red) are shown. C, Summary of current densities (pA/pF at 60 mV) at pH 6.4, 7.4, and 10.4 (n=4–16 cells per pH bar). D, KCNK3 current activity is depicted for WT (black curve), V221L (red curve), and WT−V221L heterodimer (blue curve), at extracellular pH 5.0 through 10.4, with current normalized to max current at pH 10.4 (n=5–33 cells per pH value plotted; fitted by the Hill equation). E, Scatterplot of current at pH 7.4 normalized to current at pH 10.4, for WT, V221L, WT−V221L heterodimer, and WT+V221L co‐expression. Each dot represents an independent cell recording. Mean current in each condition is displayed at the horizontal line (n=16–33 cells per condition, measured at 60 mV). Bar graphs and pH curve values show means±SEM. *P<0.05 for the comparison of WT vs WT−V221L KCNK3 dimer conditions in panel C by the unpaired Student t test; (E) P<0.05 for the comparison of all KCNK3 conditions, calculated by 1‐way ANOVA (P<0.05) and post hoc Tukey test.
