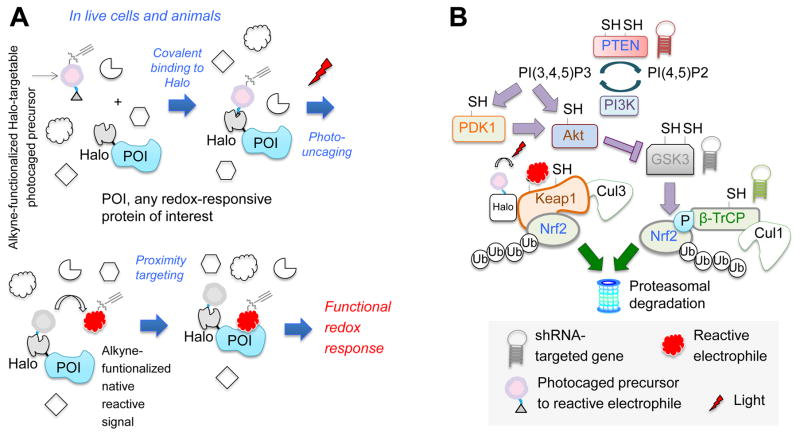
Figure 1. T-REX coupled with shRNA-knockdown investigates co-players in Keap1-alkylation-specific AR.
(A) T-REX enables a user-controlled targeted redox perturbation in vivo: photocaged precursor to reactive LDE covalently binds HaloTag fused to any POI. After washing away unbound precursor, photouncaging rapidly (t1/2 < 1 min) releases LDE. Proximity enhancement (Long, et al., 2016) facilitates selective modification of first-responding sensor-POI, unveiling on-target redox responses. (B) Model of Nrf2 regulation. “SH”s mark redox-sensitive proteins. This study probes the functional impacts of T-REX- enabled Keap1-alklylation-specific redox events in cells in which either PTEN, GSK3β, or β-TrCP (marked by “hairpins”) is depleted.