Abstract
Cancer is a complex disease that is constantly evolving. It is now the most common cause of death in Europe after cardiovascular diseases. There are inequalities among European countries, potentially unsustainable healthcare systems impacting quality of cancer care and increasing number of patients with cancer with rare conditions.
Clinical and translational research are the backbone in establishing scientific advances as novel treatments and advancing progress to the benefit of patients. Commercially sponsored clinical trials are responsible for developing new medicines that can treat various disease areas, including cancer. It is important to note, however, that these clinical trials only assess the viability of compounds that are chosen by a commercial entity that funds the entire process. By their design and focus, these trials need to fulfil commercial interests and market expectations, which do not always coincide with patients’ needs.
As soon or even before novel treatments and compounds obtain formal market authorisation, academia will take these existing and new medicines to further conduct research in order to optimise their use, develop new combinations and with a strong focus on the patients and their needs. Established standard of care most commonly relies on clinical cancer research stemming from non-commercial entities, cooperative groups or academic clinical research.
This article provides a consensus on the definition of academic research, illustrates its added value and suggests and calls to European Union institutions to support this type of research for the benefit of patients.
Keywords: independent research, academic research, cancer, legal framework
Introduction
The cancer clinical research landscape is rapidly changing, and all European Union (EU) stakeholders need to urgently adapt in order to offer patients effective and affordable cancer care.1–3 The Clinical Academic Cancer Research Forum (CAREFOR, http://www.eortc.org/carefor/) believes the EU needs to urgently increase its capacity for INDEPENDENT international academic clinical cancer research.
CAREFOR is a new, visionary multistakeholder platform that sets out to address the various funding and regulatory challenges faced by academic researchers and also to foster the advancement of collaborative cancer research by developing novel approaches to doing clinical trials, thus helping to keep Europe at the forefront of academic cancer research.
To achieve this, INDEPENDENT academic clinical cancer researchers need:
an efficient and harmonised legal framework that meets the real needs of patients and researchers;
appropriate independent funding mechanisms for international efforts.
The contribution of academic researchers to improvements of cancer care is largely documented in fields such as childhood cancers and/or haematology.4 5 More recently, the use of imatinib in soft tissue and bone sarcomas is a very good illustration of the capacity of academic research to expand the use of a novel agent to a new, rare indication that would not be necessarily explored by the drug manufacturers otherwise.6–10 Many therapeutic breakthroughs and standards of treatments have been established by academic researchers who validate complex therapeutic strategies, not necessarily limited to drugs but in combination with other treatment modalities. With its main role to optimise therapeutic strategies, academic research drives Europe's therapeutic progress and knowledge development. It has been documented that many gaps in evidence-based recommendations remain as of yet since many of the unanswered questions are of no interest for pharma companies and need to be addressed by the academia. Indeed, an analysis of 1023 recommendations found in 10 Clinical Practice Guidelines in Oncology (NCCN Guidelines) across several cancer types shows that proportions of level I category of evidence were as low as 20% for kidney, 19% for breast, 6% for lung, 9% for pancreatic, 6% for non-Hodgkin's lymphoma, 6% for melanoma, 4% for prostate and 1% for colorectal cancer.11
With personalised medicine and the arrival of multiple new technologies, we have entered an era of clinical research transformation where the role of academic research is going to be even more critical to help prioritise the appropriate treatments in an INDEPENDENT manner based on sound evidence. Many missions of importance for public health are not appropriate for the commercial sector. Therefore, CAREFOR believes academic researchers shall:
- support and accelerate the development of precision medicine by:
- ensuring INDEPENDENT research and knowledge development in Europe
- enhancing knowledge through collaborative platforms
- offering patients the most useful tests and the best information necessary to maximise their chances of gaining timely access to new clinical trials
support other stakeholders such as drug developers, regulators and health technology assessment bodies with specialised expertise, strategic vision on the unmet needs, but also through the development of new methodologies and meeting the challenge of using big data
help the society and patients to embrace new approaches such as adaptive licensing and effective implementation of new treatments, including off-label use in clinical situations of unmet need, optimising the administration of currently approved therapies, supporting outcomes research, reducing side effects, addressing surgery and radiotherapy, all by means of academic, fully independent, well-designed clinical trials.
- support initiatives aiming to:
- reduce side effects
- explore surgery and radiotherapy as adjunctive treatments
- explore population-based research and cooperation with registries.
Indeed, millions of patients with cancer and their families around the world have benefited from practice-changing discoveries made by basic and clinical cancer researchers over the last decades. Over the past 40 years, survival rates in the EU have increased significantly for many cancers.12 For example, in the 1970s, only one in four people diagnosed with cancer in the UK could expect to survive for 10 years or more, whereas currently half of patients survive their cancer for at least 10 years.13 This progress is attributable to advances in cancer research and its translation into novel treatment options and strategies through successful clinical trials, as well as to continuous efforts in the fields of cancer prevention, screening and early detection.14 15
However, there is still much to be done. The prognosis for patients with cancer has not improved equally for all cancer types, and there are still marked differences in cancer mortality across European countries. Cancer is more prevalent in the elderly so the annual number of new cases of cancer in the EU alone is expected to continue to rise from 2.6 million in 2012 to over 3.2 million by 2030.16 This is due to the increased life expectancy but also because of better detection and diagnosis. It has been forecasted that within approximately 10–15 years, in developed countries, one out of two people will have one cancer throughout their lifetime. On a global scale, the increase is even bigger with the estimated overall economic impact of cancer in 2008 already amounting to $895 billion or 1.5% of the total global gross domestic product.17
This growing burden of cancer is particularly challenging when financial constraints are being placed on the provision of quality cancer care services as well as the funding of academic cancer research. To address these and other challenges (specified further below), the Clinical Academic Cancer Research Forum (CAREFOR, http://www.eortc.org/carefor/) works to improve the situation for academic clinical trials in the field and has been established to help improve the situation for academic clinical trials in the field of oncology in Europe.
CAREFOR achieves this by reaching out to other stakeholders and by publicising the role and benefits of independent clinical research, with the ultimate goal to anchor academic cancer clinical research in the European landscape.
Affordability of cancer care and benefit of academic clinical research
The goal of commercially driven clinical cancer research is to bring profitable new drugs to market as quickly as possible, though the clinical trials undertaken may not necessarily lead to substantial increases in survival and may not always fully reflect the needs and concerns of patients. It is also clear that innovative drugs are often introduced to the market at a cost that is seldom affordable even for high-income countries, forcing healthcare systems and regulators to make particularly difficult choices. Academic cancer research, on the other hand, goes to extraordinary efforts to optimise novel therapeutic multidisciplinary strategies as well as investigating important but less commercially attractive research areas and health issues. Some examples include rare cancers, drug combinations and multimodal treatment regimens, the impact of cancer and its treatment on the human body and long-term patient follow-up.
In the new era of molecular genetics and immunotherapy, basic and translational research plays a crucial role in identifying and developing new anticancer treatments and strategies.18
Our increasing understanding of cancer biology over the last decade is leading to new forms of clinical research, such that bridging laboratory researchers to clinicians (bench to bedside) is more critical than ever. This can be achieved by maximising access to biological material in a regulatory framework that is simple and efficient and applies across the whole of Europe.
Many treatments are administered without knowing their true benefits for individual patients and often carry the risk of undue toxicity. Therefore, if personalised or precision medicine is to be achieved, it is imperative to determine the clinical relevance of treatments, whether in development or in practice, for specific groups of patients. This would have an obvious impact on healthcare budgets and can be better achieved by independent researchers, rather than industry as this is seldom its primary interest.
In fact, the knowledge on the real efficacy and ultimate potential of recently approved drugs is very limited. There is a usual need of further optimisation of the new approved drugs, with testing of different schedules and in different niches, head-to-head comparisons with other alternative therapies and other studies that may not be interesting for the owner of the drug; these trials should be accomplished by the academia.19
Non-commercial, independent cancer research, whether funded publicly or through other means, including collaboration with industries, that is, modern academic research, yields substantial economic returns in terms of improved health outcomes and has otherwise a major impact on public health. Case studies in Supplementary files 1-7 provide examples of academic research that has led to dramatic improvements in terms of quality of life and survival rates for different types of cancers; case studies also show the scale of academic research and potential impact on public health.
Independent research is endangered in Europe
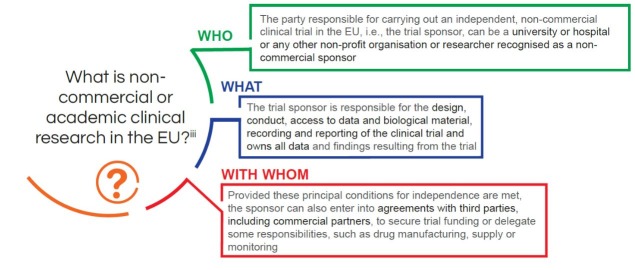
CAREFOR participants agree on the definition of academic or non-commercial research as illustrated in the figure (figure 1) below.
Figure 1 Definition of non-commercial research.
The general public knows that basic laboratory research is done by universities and other academic organisations, but it is not well known that an important part of clinical research in oncology has always been, and still is, performed by the academic sector. Critically, academic clinical research currently suffers from several unintended effects of legislation that is drafted for a specific purpose, but has a negative impact on academic research often due to a failure to recognise the key role that academic clinical research plays in supporting innovation and delivery of the high quality of the healthcare in Europe.
Data from the European Clinical Trials Database (EudraCT) actually shows that non-commercial sponsors conduct an important proportion of clinical trials (figure 2).
Figure 2 Clinical trials run by non-commercial sponsors.
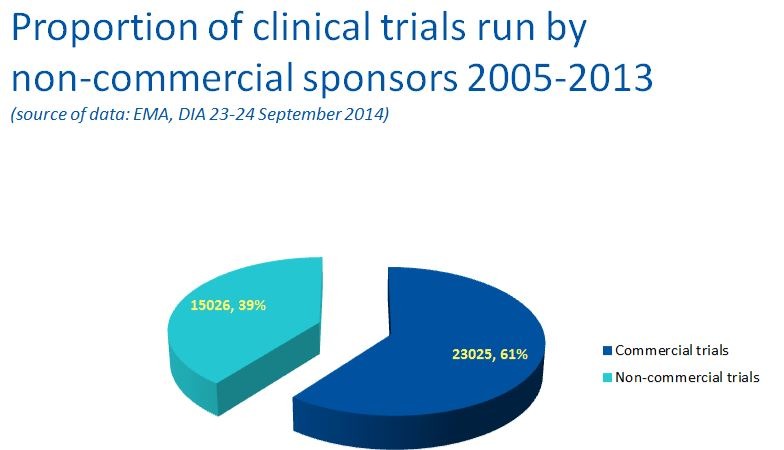
This figure does not provide a full overview of clinical research run by academia. Indeed, in oncology, drugs alone do not always provide the solution, as many cancers are managed with surgery and radiotherapy. Progress in these areas can only be achieved through clinical research conducted almost exclusively by academia. Hence, it could be argued that the overall contribution of academia to clinical research would be much higher. No global statistics exist in Europe, but based on the clinical trials register ‘ClinicalTrials.gov’, only 35% of cancer projects registered are funded by industry, with 65% being supported by health systems, government funds, universities and other research organisations.
A glance at the European Medicine Agency (EMA) public reports on clinical trial statistics shows that the proportion of non-commercial trials has recently dropped to just 20%–21% of trials.20 This is clearly a major cause for concern and may have several different explanations, including, at least for oncology, a late effect of the EU Clinical Trials Directive. However, it clearly shows a dangerous trend that will ultimately prove detrimental to cancer care.
Many of the academic clinical trials carried out in Europe have had a major positive impact on the way cancer is treated.
Funding of international efforts
Yearly investments in research of Horizon 2020, aiming to support collaborative international research initiatives, barely represent about 4% of overall investments by all EU member states. National funding mechanisms, on the other hand, prioritise national initiatives and are not fairly distributed, making international initiatives cumbersome or even impossible to fund.
There is a need to put in place an efficient mechanism of communication with National and EU funders in order to objectively evaluate needs of national versus international funding in cancer clinical research and to adapt existing mechanisms consequently.
Collaboration for patient-centred innovation
With the steadily growing number of cancer subtypes, each with its own specific molecular and genetic signature, academic clinical cancer research has an increasing role to play in developing tailored treatment options (precision medicine) on the one hand and addressing real-life implementation of therapeutic strategies on the other. New approaches and methodologies are being developed to address these two major poles through new clinical cancer research partnerships that must be facilitated and supported by policy-makers.
Independent collaborative research platforms are much better suited than commercial clinical research silos for identifying the right patients for access to new drug trials and for optimising knowledge development. These platforms can serve as a point for cancer patient access, based on molecular characteristics to enter clinical trials for ‘adaptive licensing’, a staggered drug approval process that allows a defined subgroup of patients with high medical need to access novel treatments sooner.
These platforms would form a precompetitive scheme for matching of the right tumour type with the right investigational drug before competitive drug development, while maximising knowledge about patients whose tumours may not exhibit the relevant features. This means a company would not have to screen thousands of patients to find the few hundred needed for their drug development, thus saving time and money and increasing chances for patients with rarer cancer subtypes to participate in clinical trials.
For this to happen, it is important that biological materials required for research are stored by independent collaborative platforms in non-proprietary quality controlled biobanks with transparent governance and biomaterial sharing policies, committed to the principle of partnership for drug development, rather than by commercial entities. This would help facilitate and accelerate regulatory acceptance with regard to the validation of relevant research targets and biomarkers. An example of such a collaborative platform is the EORTC SPECTAcolor (http://spectacolor.eortc.org/), a prospective, fully annotated tumour sample bank and biomarker analysis platform for the genetic profiling of patients with advanced colorectal cancer. Other groups also have examples of similar platforms (ie, AURORA project, http://www.bigagainstbreastcancer.org/projects/metastatic-breast-cancer-gps/).
High-quality collaborative research platforms will likely become the new research model (currently referred to as benchmarking studies) as they offer an effective approach when multiple (randomised) clinical trials addressing a variety of possible drug combinations are no longer feasible. These platforms will help regulators and industry as academia will function as an active partner in the drug registration process, switching from a traditional drug-centred research and approval process to a truly patient-centred approach. The aim is to find the trial fit for the patient, rather than finding the patient fit for the trial.
New methodologies for evidence generation and evaluation
Even though collectively rare cancers account for about one-fifth of new cancer cases, they pose a particular challenge to clinical cancer research. Given that low patient numbers often limit the industry's capacity to develop drugs alone for most rare cancers, in principle, any piece of new evidence would need to be exploited to help optimise the low number of patients liable to be enrolled in clinical studies on rare cancers.21
This patient population is typically willing to accept higher levels of uncertainty, so regulatory agencies and local health systems should allow methodologies to be refined. This includes using Bayesian logic, preclinical evidence, uncontrolled studies, observational evidence and analysis of retrospective cases and even anecdotal cases as well as the results of large and small randomised clinical trials.
Adaptive clinical trials in general, with their inherent potential of flexibility when properly applied, should be considered, and surrogate end points could replace clinical end points (eg, progression-free survival or tumour response) without affecting patient safety. New treatments could be explored temporarily, under the assumption that the surrogate end point is valid, while awaiting the final results. The compassionate and off-label use of drugs should be exploited as well as flexible regulatory innovations such as adaptive licensing to offer new agents early on to patients with rare cancers, while, at the same time, generating evidence.
In addition, electronic patient records for measuring the effectiveness of treatments via patient-reported outcomes and also innovative partnerships with cancer registries could be used to acquire evidence and create external controls for future studies (when internal controls are impractical) to study the effectiveness of new treatments in the real world as distinct from the artificial world of classical clinical trials. While the commercial sector will not have access to cancer registry data, academia can both help generate such information, establish collaborations and provide independent evaluation to regulators of the medical and economical added value of novel treatments in real life.
Barriers to academic clinical cancer research
A recent survey confirmed that lack of funding continues to be the most significant barrier to academic clinical cancer research around the world, irrespective of the economic circumstances of the country.22 There are many examples of projects addressing key public health questions that could not be done or that have been significantly delayed because of lack of funding. In addition, academic cancer clinical researchers in Europe are confronted by a myriad of rules and regulations that hamper successful research in practice, without any evidence that they achieve their stated goal of improving patient safety. The case studies of trials that could not be done because of regulations and/or lack of funding are presented in the Supplementary file 7. The new EU Clinical Trials Regulation (CTR)23 is likely to come into effect in 2018 having had the laudable goal of reducing the burden of bureaucracy that has been imposed by the former EU Clinical Trials Directive 2001/20/EC stifling clinical research in Europe for many years resulting in a 25% decline in the number of clinical trials conducted in the EU between 2007 and 2011.24
esmoopen-2017-000187supp007.jpg (634.6KB, jpg)
Several CTR-related measures suggest that conducting clinical trials may become easier in the future, such as the upgrading of the centralised EU clinical trials portal for increased data transparency and communication, fewer regulatory requirements for ‘low‐intervention clinical trials’ and ‘one-time consent’ from patients for the use of their data and biomaterial for research outside the clinical trial. However, the CTR still has to be fully implemented at the national level in a harmonised fashion across Europe, and stakeholders still have to be properly informed and educated.
CAREFOR welcomes more recent finalisation of the General Data Protection Regulation
(GDPR). It is of crucial importance for academic clinical cancer research that data protection rules in Europe enable healthcare researchers and their partners to optimally use certain types of personal data. This data can support, improve and preserve the quality of healthcare and also foster innovation within the EU without jeopardising patient confidentiality. CAREFOR specifically welcomes research being acknowledged as a lawful purpose for data processing and for allowing information on future research to be less specific provided patient consent is unambiguous. This principle is also embedded in the CTR and repeated throughout as an absolute prerequisite for fostering clinical research in the EU by academia.25 26 New regulations also recognise the importance of registries and that new consents may not always be required for the secondary use of data. Furthermore, it recognises the value of pseudonymisation as being a potentially valid means of safeguarding patient anonymity, and it enables each union or member state to further specify additional derogations.
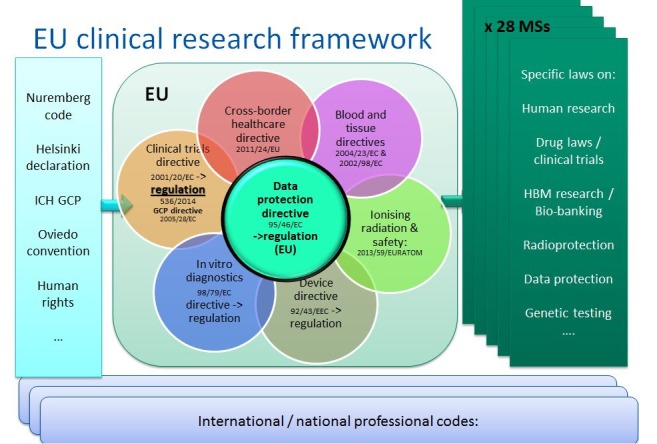
As stated earlier, both CTR and GDPR still need to be implemented in the EU as a whole and, despite both being regulations, elements of both need to be incorporated into national law, thus maintaining heterogeneity of legal frameworks in EU. Moreover, they are a very good example of the piling up of regulations developed without any in-depth analysis of the full impact they might have on a single research project. The complexity of the current legal framework, as illustrated in the figure below, renders the EU less attractive for industry investment and limits the amount and type of research academics are able to do. This further weakens research and inhibits innovation in the EU to such an extent that it presents a challenge to the fundamental right of EU citizens to undertake research (figure 3).
Figure 3 EU clinical research framework.
Conclusion
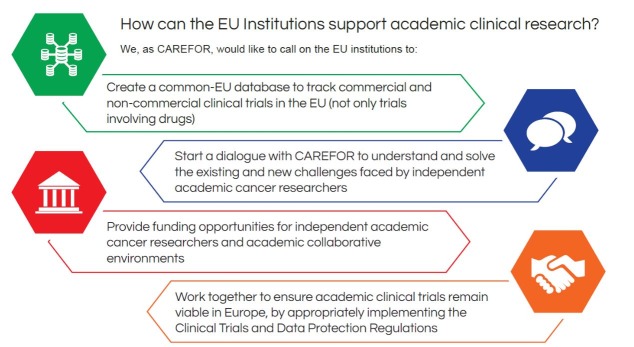
In conclusion, CAREFOR believes EU institutions shall urgently work on ways to clinical research in the fourfold way, as illustrated below (figure 4).
Figure 4 How can the EU institutions support the academic clinical research?
CAREFOR is committed to work closely with the EU parliament, the European Commission and Member States to ensure EU is equipped with efficient legal framework and visionary that make EU the best place for fostering research and innovation in general and more specifically in the field of cancer clinical academic research.
CAREFOR will specifically ensure that the framework for pan-EU clinical research whether interventional or non-interventional is pragmatic and sound to allow biobanks to function and permit the full use of data and samples collected. It should also avoid unnecessary administrative obstacles and unwarranted costs, be in tune with the rapidly changing research landscape and meet the need for collaboration, partnership, data sharing and transparency.
CAREFOR will carefully monitor and document the need for efficient independent clinical cancer research in order to provide EU regulators and policy-makers with strong expert opinion and constructive suggestions.
CAREFOR: joining efforts to foster academic clinical cancer research
To address the lack of awareness on the importance of academic clinical research, three leading European organisations—the European Association for Cancer Research, the European Organisation for the Research and Treatment of Cancer (EORTC), and the European Society for Medical Oncology (ESMO)—have established the Clinical Academic Cancer Research Forum (CAREFOR, http://www.eortc.org/carefor/) in 2014. The annual forum brings together cancer groups and networks as well as individual experts, patients and other relevant stakeholders to encourage and support innovative academic clinical research in oncology. CAREFOR seeks to address the aforementioned and additional challenges faced by all those involved in conducting academic research and to find suitable solutions.
Topics that will be addressed at this forum include securing funding for academic clinical trials, establishing rules of collaboration with industry, identifying the implications of cross-border collaboration, involving patients in clinical trials, guaranteeing data protection and transparency and fostering the freedom of research while implementing the CTR.
CAREFOR therefore reflects the willingness of academia to work together towards supporting the EU mission of bringing the constantly evolving group of diseases that comprise cancer under control and in providing patients with a better quality of life and the hope for a cure. Academic clinical trials form the cornerstone of such research and, as such, deserve safeguarding and fostering. CAREFOR welcomes an open and constructive dialogue with European policy-makers to facilitate academic clinical trials in Europe and to keep Europe at the cutting edge of cancer research.
About the European Association for Cancer Research
The EACR is Europe's largest professional member society for cancer researchers with over 9500 members worldwide. We provide a wide range of services to members, facilitate communication and collaboration and organise a series of cancer research conferences where the latest research topics and interaction are the highest priorities.
About the European Organisation for the Research and Treatment of Cancer
Created in 1962, the EORTC is a non-profit international cancer research organisation under Belgian Law. The EORTC is conducting multidisciplinary international cancer clinical research in a network of over 300 university hospitals located in 32 countries in which some 2900 clinicians and scientists collaborate on a voluntary basis. Over 40 EORTC clinical trials are open to patient recruitment at any given time. The EORTC operates on an independent basis, and in this capacity is able to work in partnership with the pharmaceutical industry in evaluating innovative molecules. EORTC has contributed to several success stories of new anti-cancer drug development including registrations by the Food and Drug Administration and the EMA. The EORTC has proven track records in establishing new standards, for example, Response Evaluation Criteria In Solid Tumors (RECIST), Quality of Life, and so on. The EORTC is involved in various EU-funded projects. See www.eortc.org for more information.
About the European Society for Medical Oncology
The ESMO is the leading European professional organisation for medical oncology. Comprising more than 16 000 oncology professionals from over 130 countries, we are the society of reference for oncology education and information. We are committed to supporting our members to develop and advance in a fast-evolving professional environment.
About the supporting organisations
The CAREFOR initiative is supported by numerous European and international organisations, patient advocacy groups, umbrella organisations, societies, cooperative groups and numerous cancer centres that have greatly contributed to healthcare improvement and management of patients in the field of oncology (in the alphabetical order):
ABCSG, ACR-ITR, AGO, ANOCEF, ARCAGY GINECO, ASST, BIG, CML Advocates Network, EACR, EORTC, ESMO, ETOP, FFCD, FICOG, GCO, GEICAM, GEIS, GEMCAD, GERCOR, GOIRC, GORTEC, Hospital General Universitario Gregorio Marañón, IBCSG, IFCT, IOCN, IRCCS, JCOG, Jules Bordet Institute, LACOG, LMU, LYSA & LYSARC, MELAMONA Patient Network Europe, National & Kapodistrian University of Athens, Netherlands Cancer Institute, SAKK, SOCUG, TTD, UNICANCER, Vall d’Hebron Institut of Oncology.
See http://www.eortc.org/carefor/ for further information and list of participants.
esmoopen-2017-000187supp001.jpg (781KB, jpg)
esmoopen-2017-000187supp002.jpg (691.6KB, jpg)
esmoopen-2017-000187supp003.jpg (756.8KB, jpg)
esmoopen-2017-000187supp004.jpg (785.3KB, jpg)
esmoopen-2017-000187supp005.jpg (687.5KB, jpg)
esmoopen-2017-000187supp006.jpg (713.3KB, jpg)
Footnotes
Contributors: All authors contributed equally.
Competing interests: None declared.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1. Lacombe D, Tejpar S, Salgado R, et al. European perspective for effective cancer drug development. Nat Rev Clin Oncol 2014;11:492–8. 10.1038/nrclinonc.2014.98 [DOI] [PubMed] [Google Scholar]
- 2. Lacombe D, Burock S, Meunier F. Academia–industry partnerships: are we ready for new models of partnership? Eur J Cancer 2013;49:1–7. 10.1016/j.ejca.2012.09.027 [DOI] [PubMed] [Google Scholar]
- 3. Denis Lacombe quietly orchestrating the clinical research revolution. Cancer World 2015;68:4–10. [Google Scholar]
- 4. 50 years of haematology: Research that revolutionized patient care.
- 5. Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011;377:42–51. 10.1016/S0140-6736(10)62175-7 [DOI] [PubMed] [Google Scholar]
- 6. Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26(4):620–5. 10.1200/JCO.2007.13.4403 [DOI] [PubMed] [Google Scholar]
- 7. Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer 2003;39:2006–11. [PubMed] [Google Scholar]
- 8. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626–32. 10.1200/JCO.2007.13.4452 [DOI] [PubMed] [Google Scholar]
- 9. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. The Lancet 2004;364(9440):1127–34. 10.1016/S0140-6736(04)17098-0 [DOI] [PubMed] [Google Scholar]
- 10. Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol 2007;25(9):1107–13. 10.1200/JCO.2006.09.0183 [DOI] [PubMed] [Google Scholar]
- 11. Poonacha TK, Go RS. Level of scientific evidence underlying recommendations arising from the National Comprehensive Cancer Network clinical practice guidelines. J Clin Oncol 2011;29:186–91. 10.1200/JCO.2010.31.6414 [DOI] [PubMed] [Google Scholar]
- 12. Malvezzi M, Bertuccio P, Rosso T, et al. European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women? Ann Oncol 2015;26 786:779–86 http://annonc.oxfordjournals.org/content/26/4/779.full.pdf+html?sid=45252ba7-f147-46dc-8e1bc7d796750f69 10.1093/annonc/mdv001 [DOI] [PubMed] [Google Scholar]
- 13. Cancer Research UK. Cancer Statistics for the UK. Cancer survival for all cancers combined. 2015. http://www.cancerresearchuk.org/health-professional/cancer-statistics/survival#heading-Zero.
- 14. Sun E, Jena AB, Lakdawalle D, et al. The contributions of improved therapy and earlier detection to cancer survival gains, 1988–2000. Forum Health Econ Policy;1 13(2)Article http://works.bepress.com/cgi/viewcontent.cgi?article=1077&context=dana_goldman. [Google Scholar]
- 15. Glover M, Buxton M, Guthrie S, et al. Estimating the returns to UK publicly funded cancer-related research in terms of the net value of improved health outcomes. BMC Med 2014;12 http://www.biomedcentral.com/content/pdf/1741-7015-12-99.pdf 10.1186/1741-7015-12-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://globocan.iarc.fr/Pages/burden_sel.aspx. Page 13 of 20.
- 17. Society AC. The global economic cost of cancer. American Cancer Society 2010. http://www.cancer.org/acs/groups/content/@internationalaffairs/documents/document/acspc-026203.pdf. [Google Scholar]
- 18. Yarden Y, Caldes C. Basic cancer research: why it is essential for the future of cancer therapy. Eur J Cancer 2013;49 2620:2619 http://eacr.org/news/2014/editorial/22%2023%20Position%20Paper.pdf. [DOI] [PubMed] [Google Scholar]
- 19. Díaz-Rubio E, Gómez-España A, Massutí B, et al. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist 2012;17:15–25. 10.1634/theoncologist.2011-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EudraCT Public Web Report for July 2015. (https://eudract.ema.europa.eu/docs/statistics/EudraCT_Statistics_2015/EudraCT_Public_Report_July_2015.pdf)
- 21. Casali PG, Bruzzi P, Bogaerts J, et al. Rare Cancers Europe (RCE) Consensus Panel. Rare Cancers Europe (RCE) methodological recommendations for clinical studies in rare cancers: a European consensus position paper. Ann Oncol 2015;26:300–6 http://annonc.oxfordjournals.org/content/26/2/300.full.pdf+html 10.1093/annonc/mdu459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seruga B, Sadikov A, Cazap EL, et al. Barriers and challenges to global clinical cancer research. Oncologist 2014;19:61–7 http://theoncologist.alphamedpress.org/content/19/1/61.full.pdf+html 10.1634/theoncologist.2013-0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC. http://eurlex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2014.158.01.0001.01.ENG.
- 24. Fostering EU's attractiveness in clinical research: Commission proposes to revamp rules on trials with medicines. Press release 2012;17 http://ec.europa.eu/health/files/clinicaltrials/2012_07/pressreleases/ip-12-795_en.pdf. [Google Scholar]
- 25. Dittrich C, Negrouk A, Casali PG. European Society for Medical Oncology (ESMO) Switzerland, and the European Organisation for Research and Treatment of Cancer (EORTC), Belgium. An ESMO-EORTC position paper on the EU clinical trials regulation and EMA's transparency policy: making European research more competitive again. Ann Oncol 2015;26:829–32 http://annonc.oxfordjournals.org/content/26/5/829.full.pdf+html 10.1093/annonc/mdv154 [DOI] [PubMed] [Google Scholar]
- 26. Casali PG. European Society for Medical Oncology (ESMO) Switzerland. Risks of the New EU Data Protection regulation: an ESMO position paper endorsed by the European Oncology community. Ann Oncol 2014;25:1458–61 http://annonc.oxfordjournals.org/content/25/8/1458.full.pdf+html 10.1093/annonc/mdu218 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2017-000187supp007.jpg (634.6KB, jpg)
esmoopen-2017-000187supp001.jpg (781KB, jpg)
esmoopen-2017-000187supp002.jpg (691.6KB, jpg)
esmoopen-2017-000187supp003.jpg (756.8KB, jpg)
esmoopen-2017-000187supp004.jpg (785.3KB, jpg)
esmoopen-2017-000187supp005.jpg (687.5KB, jpg)
esmoopen-2017-000187supp006.jpg (713.3KB, jpg)