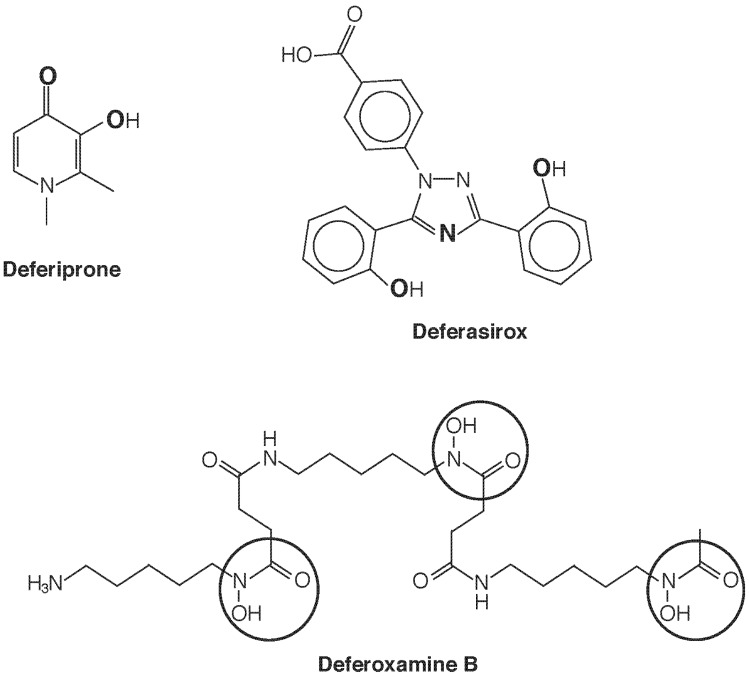
Figure 4.
Iron chelating agents in clinical use. Deferiprone chelates Fe3+ with 3:1 stoichiometry in a bidentate fashion upon deprotonation of the hydroxyl group, using the two O atoms shown in bold. Deferasirox is a tridentate Fe3+ chelator that, upon deprotonation of the two phenolic groups, uses the three bold atoms (two O and one N) to bind in a 2:1 complex. Hexadentate deferoxamine B forms a 1:1 complex with Fe3+ using the six O atoms of the three circled hydroxamate groups.