Table 2. Investigator‐assessed BOR and PFS for all patients and for predefined subgroup analyses stratified by prior trastuzumab treatment and hormone receptor status (intent‐to‐treat population).
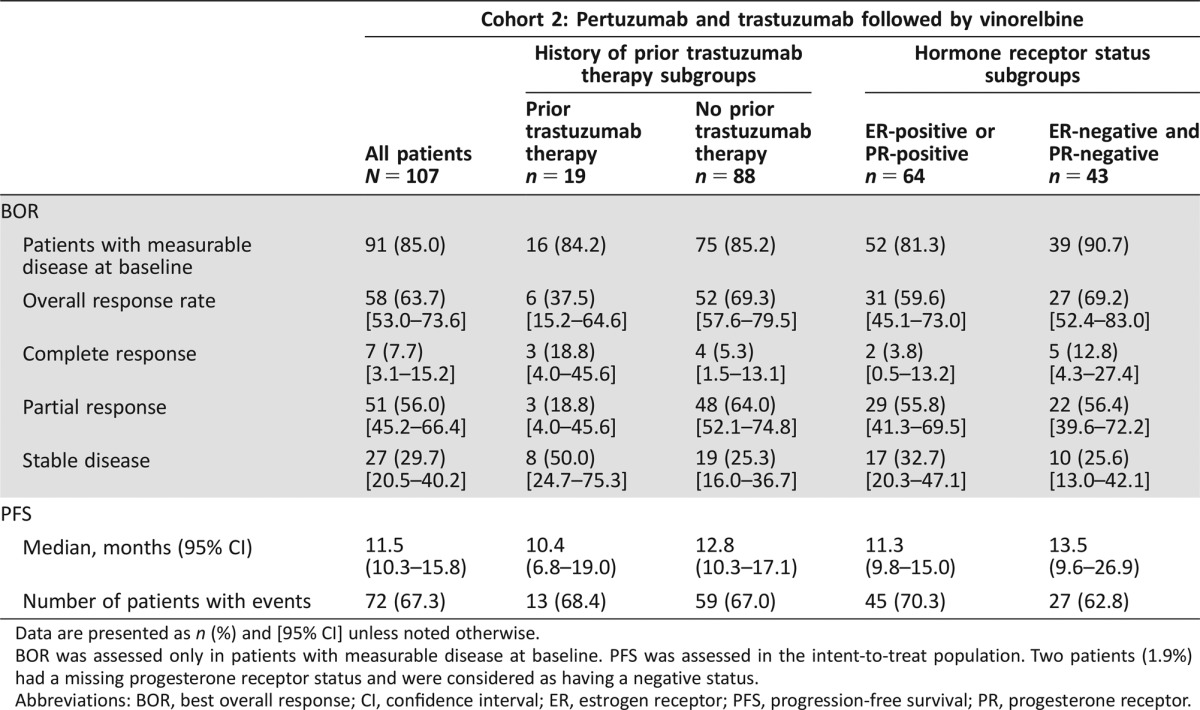
Data are presented as n (%) and [95% CI] unless noted otherwise.
BOR was assessed only in patients with measurable disease at baseline. PFS was assessed in the intent‐to‐treat population. Two patients (1.9%) had a missing progesterone receptor status and were considered as having a negative status.
Abbreviations: BOR, best overall response; CI, confidence interval; ER, estrogen receptor; PFS, progression‐free survival; PR, progesterone receptor.
