Abstract
Congenital heart disease is a leading cause of death in the newborn period, and man-made grafts currently used for reconstruction are associated with multiple complications. Tissue engineering can provide an alternative source of vascular tissue in congenital cardiac surgery. Clinical trials have been successful overall, but the most frequent complication is graft stenosis. Recent studies in animal models have indicated the important role of the recipient’s immune response in neotissue formation, and that modulating the immune response can reduce the incidence of stenosis.
Keywords: tissue engineering, tissue-engineered vascular graft, congenital cardiac disease, surgery, Fontan, bone marrow-derived mononuclear cells
1. Introduction
Congenital cardiac anomalies are the most common birth defect, affecting nearly 1% of all live births. [1] Of these patients, one-quarter have severe disease and will ultimately require major reconstructive surgery during their lifetimes. [2] Although significant improvements have been made in the management of congenital heart defects in recent decades, they remain a leading cause of death in the newborn period. [3] A substantial portion of the morbidity and mortality of pediatric cardiac surgery arises from the synthetic conduits and patches frequently used to repair congenital defects. These man-made grafts, which are constructed of materials such as polytetrafluoroethylene (PTFE, or Gore-Tex®), are susceptible to thromboembolism, stenosis, ectopic calcification, and infection. [4,5] They also lack growth potential, contributing to one of their greatest sources of morbidity in the pediatric population: somatic overgrowth, or the process by which patients outgrow their grafts. Allografts, xenografts, and autologous tissues such as pericardium and saphenous vein have been used as alternatives, but all are associated with similar complications to varying degrees, and none have growth potential. Graft failure rates have been reported to be 70 to 100% at 10 years. [5,6] Patients therefore require serial reoperations to replace their failed grafts, each of which is associated with its own morbidity and mortality.
Vascular tissue engineering provides a potential solution to this problem. Rather than using a synthetic material, a biodegradable scaffold is implanted and degrades over time, replaced with autologous vascular tissue that can repair, remodel, and even grow with the patient. [7] Because the neovessel is composed entirely of autologous tissue, it is theoretically not plagued by the complications associated with synthetic materials. Classically, cells from the recipient are seeded onto the graft prior to implantation. In addition to the scaffold itself, these seeded cells play a crucial role in the process of neotissue formation. In this review, we present the current status of tissue engineered vascular grafts (TEVGs) for use in congenital cardiac surgery and our experience implanting TEVGs seeded with bone marrow-derived mononuclear cells.
2. Congenital heart disease and the demand for tissue
Approximately 25% of patients with congenital heart disease have critical defects, which are a subset that require surgical or transcatheter intervention before one year of age. [2] The traditional paradigm for these patients was early palliation followed by definitive correction later in life. With advancements in surgical technique and perioperative critical care, however, that strategy has shifted to one of early repair, even in small infants. [8] In several critical congenital heart defects, transcatheter interventions are an emerging option, providing an alternative to open surgery and its associated complications.
Critical congenital heart defects have traditionally been divided into cyanotic and non-cyanotic lesions, but with the aforementioned advancements in surgical care, some have advocated for a classification into (1) defects which should be repaired, (2) those which must be palliated, and (3) a final group in which either option is acceptable. [8] A list of critical congenital heart defects, their pathophysiology, and surgical options is summarized in Table 1. The most common critical congenital heart defects are ventricular and atrial septal defects, which are two of a limited number of defects which may be monitored in certain patients. [1] Most other critical defects require repair or palliation irrespective of any other criteria. [8]
Table 1.
Critical congenital heart defects and surgical interventions. Most critical congenital heart defects require repair or palliation, and many of the procedures use additional tissue in the form of autologous or synthetic grafts, patches or valves. [8] Tissue-engineered constructs have the potential to replace these grafts and form autologous structures which can repair, remodel, and grow with patients.
| Defect | Description | Pathophysiology | Indications for intervention | Most common interventions | Need for additional tissue |
|---|---|---|---|---|---|
| Defects in which repair is the best option | |||||
| Aortic coarctation | Luminal narrowing of aorta, most commonly distal to left subclavian artery | LV outflow obstruction, pulmonary overcirculation, biventricular failure, systemic hypertension | Hemodynamic changes secondary to defect |
|
Sometimes |
| Aortic stenosis | Obstruction of LVOT | LV hypertrophy and ischemia, congestive heart failure | Transvalvular gradient of 50 mmHg with associated symptoms or EKG evidence of LV strain or ischemia |
|
Sometimes |
| Aortopulmonary window | Incomplete development of septum that normally divides truncus into aorta and PA | Left-to-right shunt, pulmonary overcirculation, congestive heart failure | Presence of defect | Pulmonary artery closure with patch, +/− additional patch repair of aortic defect | Yes |
| Atrial septal defect (ASD) | Opening in interatrial septum | Left-to-right shunt, RV volume overload, RV hypertrophy |
|
|
Sometimes |
| Cor triatriatum | Fibromuscular diaphragm divides LA into superior chamber (drainage from pulmonary veins) and inferior chamber (communicates with mitral valve and LV), +/− ASD | Obstruction of pulmonary venous return, low cardiac output, pulmonary venous hypertension | Presence of defect | Surgical excision of membrane, ASD closure | Yes |
| Patent ductus arteriosus (PDA) | Failure of closure of ductus arteriosus (shunt from PA to descending aorta) | Left-to-right shunt, LV volume overload, RV strain | Presence of defect |
|
No |
| Total anomalous pulmonary venous connection | Abnormal drainage of pulmonary veins into right heart‡ | Pulmonary overcirculation | Presence of defect | Anastomosis of common pulmonary venous channel to LA, obliteration of anomalous venous connection, ASD closure | Yes |
| Truncus arteriosus | Single great artery arises from heart and supplies pulmonary, systemic, and coronary circulations | Left-to-right shunt, congestive heart failure | Presence of defect | Separation of pulmonary arteries from aorta, closure of aortic defect (+/− patch), graft reconstruction of RVOT, VSD closure | Yes |
| Defects requiring palliation | |||||
| Hypoplastic left heart syndrome | Varying underdevelopme nt of left heart structures, including LV, aortic and mitral valves | Pulmonary venous hypertension, pulmonary overcirculation, RV failure | Presence of defect | Three-stage palliation ending in variation of Fontan§ | Yes |
| Tricuspid atresia | Atresia of tricuspid valve, usually with associated ASD | RV hypoplasia, severe cyanosis upon ductus arteriosus closure‖ | Presence of defect | Fontan and its modifications¶ | Sometimes |
| Defects which may be repaired or palliated | |||||
| Atrioventricular (AV) canal defects | Failure of fusion of endocardial cushions in central heart | Left-to-right shunt, valvular regurgitation, congestive heart failure | Presence of defect |
|
Yes |
| Double-outlet right ventricle | Aorta and PA both arise entirely or partially from RV, often with VSD | Cyanosis, pulmonary hypertension, congestive heart failure | Presence of defect | Surgical relief of pulmonary stenosis, outflow from correct ventricle to correct great artery, and separation of systemic and pulmonary circulations | Sometimes |
| Ebstein’s anomaly | Inferior displacement of tricuspid valve into RV** | RV inflow obstruction and tricuspid regurgitation, ineffective RV filling, RV contractile dysfunction, pulmonary atresia secondary to lack of forward flow |
|
|
Sometimes |
| Interrupted aortic arch | Absence of luminal continuity between ascending and descending aorta, usually with VSD or PDA | Metabolic acidosis and hemodynamic collapse upon ductus arteriosus closure | Presence of defect |
|
Sometimes |
| Tetralogy of Fallot | VSD, overriding aorta, RVOT obstruction, RV hypertrophy | RVOT obstruction, RV hypertrophy, worsening RVOT obstruction, extreme cyanotic spells | Presence of defect |
|
Yes |
| Transposition of great arteries | Aorta arises from RV, PA arises from LV | Parallel pulmonary and systemic circulations, survival dependent on intracardiac mixing with left-to-right shunt | Presence of defect | Sometimes | |
| Ventricular septal defect (VSD) | Opening in interventricular septum | Left-to-right shunt, pulmonary overcirculation, congestive heart failure, pulmonary hypertension, reversal to right-to-left shunt |
|
Patch repair (open or percutaneous) | Yes |
Transposition of the pulmonary valve into the aortic position with reconstruction of pulmonary outflow tract.
Routine repair is advocated by some due to the risk of paradoxical embolism with an ASD.
Must have right-to-left intracardiac shunt (ASD) to be compatible with life.
In stage 1 (modified Norwood procedure), the LV is bypassed by creating a single outflow vessel, the neoaorta, which arises from the RV. A graft is used to augment the native aorta. In stage 2, a bidirectional cavopulmonary shunt (bidirectional Glenn) is created to begin to separate the pulmonary and systemic circulations. In stage 3, a modified or extracardiac Fontan is performed.
Pulmonary blood flow dependent on a PDA unless there is an associated VSD.
The original Fontan procedure consisted of a pulmonary artery-to-superior vena cava anastomosis (Glenn), ASD closure, direct connection of RA to the proximal left pulmonary artery, ligation of the main pulmonary artery, and implantation of a homograft valve into the orifice of the inferior vena cava. The modified Fontan operation uses an interatrial lateral tunnel to route inferior vena caval blood directly to the superior vena cava. In the fenestrated Fontan repair, a residual 20–30% right-to-left shunt is created or left unrepaired to sustain systemic output in the setting of elevations in pulmonary vascular resistance postoperatively. In the extracardiac Fontan, an extracardiac graft is used as a conduit to channel inferior vena caval blood to the pulmonary arteries. The Fontan and its modifications are usually performed in a staged manner, with patients receiving small systemic-to-pulmonary shunts early in life, followed by larger operations in preparation for a Fontan.
RV is divided into an inlet portion, which is atrialized, and an outlet portion, which is the true or trabeculated RV.
Superior vena caval blood is diverted directly to pulmonary arterial system by a bidirectional cavopulmonary shunt, and RV pumps inferior vena caval blood to pulmonary arteries via the RVOT.
The Senning repair realigns the atrial septum over the pulmonary veins and uses the right atrial free wall to create a pulmonary venous baffle. In the Mustard repair, autologous pericardium or a synthetic material is used to create the interatrial baffle.
Division of aorta and pulmonary artery, posterior translocation of aorta, mobilization of coronary arteries, placement of pericardial patch, and realignment of coronary arteries on neoaorta.
LA, left atrium; LV, left ventricle; LVOT, left ventricular outflow tract; NYHA, New York Heart Association; PA, pulmonary artery; Qp:Qs, pulmonary-to-systemic flow ratio; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract
As indicated in Table 1, critical congenital heart defects are characterized by an absence or malformation of normal cardiac tissue during development. Many surgical reconstructions therefore require a supplemental source of tissue. Ventricular septal defects and large atrial septal defects are closed with patches. Long aortic coarctations require patch aortoplasties or interposition grafts. Aortic stenosis can be managed by the Ross procedure, which uses the native pulmonary valve to replace the stenotic aortic valve, with the right ventricular outflow tract then reconstructed with a valved conduit. [8] In contrast to coronary artery bypass and arteriovenous grafts for dialysis access, which are common uses of tubular grafts in adult cardiovascular surgery, most grafts in congenital heart disease are implanted entirely or partially in the lower-pressure venous circulation. In addition, the target graft diameter is larger than in the comparatively narrow coronary circulation.
This contrast is apparent in the Fontan procedure, in which a 2-cm diameter graft is implanted in a low-pressure system to palliate single-ventricle anomalies. It routes vena caval blood directly to the pulmonary circulation, with the single functional ventricle then pumping oxygenated blood systemically. [9] In the original description by Fontan in 1971, the venous blood was directed to the pulmonary circulation via an anastomosis between the right atrium and the right pulmonary artery. [9] Over the following decades, the procedure was altered in several ways. In the modified Fontan procedure, an interatrial lateral tunnel directs the blood to the superior vena cava. The superior vena cava is ligated, with the superior end sutured to the upper right pulmonary artery and the inferior portion to the underside of the right pulmonary artery. [8] Venous blood then flows passively and laminarly to the pulmonary circulation.
A further modification is the extracardiac Fontan, in which a prosthetic graft is used to connect the inferior vena cava to the pulmonary artery (Figure 1). By utilizing an extracardiac conduit, this procedure does not alter the native atrial geometry, and it maximizes laminar flow. [8] Compared to other variations of the Fontan, the extracardiac approach has a lower incidence of arrhythmias, cavopulmonary pathway obstructions, re-interventions, and late deaths. [4] However, the implantation of prosthetic grafts without growth potential means surgeons must wait for patients to grow to a size where graft implantation is possible, at which time they frequently implant oversized grafts to account for future additional patient growth.
Figure 1.
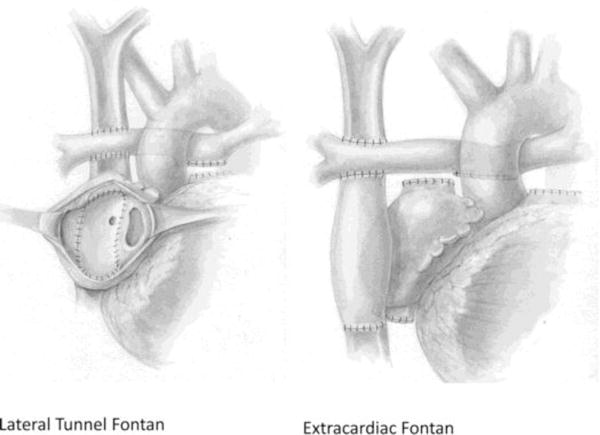
The Fontan circulation. In a lateral tunnel Fontan (left), a baffle within the right atrium directs blood from the inferior vena cava to the pulmonary artery. In an extracardiac Fontan (right), a prosthetic graft is used to route blood directly from the inferior vena cava to the pulmonary artery. Many outcomes are better after the extracardiac variant, but it currently requires the use of a synthetic graft without growth potential. Tissue-engineered vascular grafts are an alternative source of tissue.
Reused with permission from: Kogon B. Is the extracardiac conduit the preferred Fontan approach for patients with univentricular hearts? The extracardiac conduit is the preferred Fontan approach for patients with univentricular hearts. Circulation. 2012;126(21):2511–2515. http://circ.ahajournals.org/
Prosthetic grafts have thus been used in the Fontan procedure with acceptable outcomes, but there remains room for improvement. The grafts are implanted in a high-flow system, which theoretically reduce the risk of thrombosis, and are subjected to low pressures, which decrease the chance of aneurysmal dilation. For these reasons, the Fontan procedure represents an ideal starting point for the investigation of TEVGs. Nevertheless, single-ventricle anomalies are rare relative to other types of critical congenital heart defects. [1] In the long term, tissue engineering has the potential to be applied to all situations in which prosthetic grafts are used in congenital cardiac surgery, including synthetic patches and heart valves. However, patches are frequently exposed to high arterial pressures, and functional heart valve leaflets are more complex to create than tubular blood vessels. As a result, the TEVG field is closer to clinical application than tissue-engineered patches and valves. This review will therefore focus primarily on the use of TEVGs for critical congenital heart defects.
3. Tissue engineered vascular graft scaffolds as structures for neotissue formation
3.1. Principles of vascular tissue engineering
Vascular tissue engineering is governed by the tissue engineering triad, which consists of three essential pillars: (1) the scaffold, which ultimately degrades and is replaced by extracellular matrix, (2) cells (either seeded in vitro or mobilized in vivo), and (3) signals (humoral and mechanical). [10] The three factors are interdependent and are all required for the formation of organized vascular tissue. Various combinations of the components have been intermixed throughout the past two decades in an attempt to create the optimal neovessel.
3.2. Scaffold types
The scaffold provides a three-dimensional structure onto which neotissue can grow. In vascular tissue engineering, two general types of scaffold have been used: (1) decellularized extracellular matrix (ECM) and (2) biodegradable synthetic materials. Decellularized tissue sources include allogeneic, xenogenic, or in vitro-engineered tubular tissues that contain an intact ECM such as human skin or vein, bovine or porcine carotid vessels, and other tissue-generated grafts. [11] Decellularization can be achieved by a combination of physical agitation, chemical surfactant removal, and enzymatic digestion, which disrupt cells and remove most of the cellular antigenic components. However, the decellularization process can also degrade matrix components, which results in a loss of ECM integrity. The resultant tissue deterioration can lead to degenerative structural graft failure. [12] Additional drawbacks of decellularized scaffolds include an inability to modify the matrix content and architecture, rapid degradation, and a risk of viral transmission upon implantation.
Alternatively, naturally-occurring or synthetic polymers can be used to form scaffolds for TEVGs. The ideal scaffold allows for a microenvironment that promotes cell adhesion and differentiation and permits deposition of ECM. Natural materials include collagen, gelatin, hyaluronate, glycosaminoglycan, chitosan, alginate, silk, fibrin, dextran, and Matrigel. [13] Synthetic polymers include polyglycolic acid (PGA), polylactic acid (PLA), polylactic-co-glycolic acid (PLGA), poly-L-lactic acid (PLLA), poly-ε-caprolactone (PCL), polyethylene glycol (PEG), polyvinyl alcohol (PVA), polypropylene fumarate (PPF), and polyacrylic acid (PAA). [13] Scaffold selection depends on biocompatibility, mechanical properties, and biodegradability.
Different materials have been used as scaffolds in vascular tissue engineering. We began by using a PGA scaffold and later added a 50:50 copolymer blend of polycaprolactone and polylactic acid (PCLA). [14] The scaffold can be fabricated in a range of sizes, typically measuring 13 cm in length, 1.5 mm in thickness and 10–24 mm in diameter. The matrix is porous, with a pore diameter of 100–200 μm. The scaffold loses its biomechanical integrity within eight weeks, but complete degradation requires six months.
4. Vascular cell types and the importance of cell seeding
Regardless of the type of scaffold, the goal remains the same: to permit cellular deposition and then degrade, thereafter providing an autologous structure for ongoing TEVG growth. The components of native blood vessels include ECM, smooth muscle cells, fibroblasts, and endothelial cells. The ideal TEVG would approximate the composition of the native vasculature.
4.1. Extracellular matrix
Extracellular matrices are complex, three-dimensional networks of proteins and proteoglycans. They have many functions: (1) they determine the biomechanical properties of tissue, (2) they are the scaffold to which cells migrate and adhere, (3) they contribute to cellular phenotype, and (4) they are an anchor for many proteins, including growth factors and enzymes. [15] Collagen and elastin are the most abundant proteins in vascular ECM, and they are both structurally and functionally important for determining the biomechanical properties of the vascular wall. The amounts of collagen and elastin in the vascular wall determine biomechanical properties such as compliance, stiffness, and burst strength. The ideal TEVG would have biomechanical properties, and therefore collagen and elastin deposition, that replicate native blood vessels.
4.2. Smooth muscle cells, fibroblasts, and endothelial cells
Native blood vessel walls consist of smooth muscle cells and fibroblasts, both of which are important for ECM deposition, maintenance of functional and structural integrity, vasodilation, and vasoconstriction. The innermost layer consists of endothelial cells, which have a potent antithrombotic cell surface. They also attenuate long-term smooth muscle cell migration and proliferation and ECM production, thus preventing intimal hyperplasia. As such, the presence of endothelial cells in neotissue is one of the most important ways to maintain long-term TEVG patency. Previous reports have demonstrated that after synthetic graft implantation for peripheral arterial disease, the number of endothelial cells found on the luminal surface in synthetic grafts was less than 10% of that seen in native vessels. [16] Other studies have shown that endothelial cells have limited ability to migrate, so endothelial cell ingrowth is unlikely to occur greater than 3 cm from the anastomoses. [17]
4.3. Cell seeding
Direct endothelial cell seeding represents a potential solution to this challenge. Endothelial cell seeding onto prosthetic grafts was first proposed as a single-stage technique, but significant cell loss occurred after the grafts were exposed to pulsatile flow. [18] A two-stage strategy was subsequently developed in which autologous endothelial cells are first harvested and cultured and then seeded onto a synthetic conduit. Patency of synthetic grafts seeded in this manner was significantly greater than unseeded grafts at three years (84.7% vs. 55.4%). [19]
While endothelial cell seeding improves patency of synthetic grafts, cell seeding is perhaps even more important to the success of tissue-engineered grafts. [20] Several different seeding methods have been described, including passive and dynamic (rotational or vacuum-dependent) strategies. [21]
Similar to the aforementioned work on synthetic grafts, our initial technique consisted of biopsying a peripheral blood vessel, explanting the tissue to isolate cells, and expanding the cells in culture. Mixed culture cells consisting of endothelial cells, fibroblasts, and smooth muscle cells were seeded onto a PGA scaffold and incubated in vitro prior to implantation in an ovine model. [22] The implanted grafts remained patent at up to 24-week follow-up, confirming the feasibility of using tissue engineering to create neovessels for use in cardiac surgery. [22] Seeding a mixed cell population onto a biodegradable scaffold was similarly successful in inferior vena cava replacement in dogs. [23] There were no complications such as thrombosis, stenosis or dilation.
In 1999, we used this technique to perform the first implantation of a TEVG in a human. Cells were isolated from a saphenous vein biopsy and seeded onto a scaffold composed of a polycaprolactone-polylactic acid copolymer reinforced with woven PGA. The graft was implanted 10 days later to reconstruct an occluded pulmonary artery in a child with congenital cardiac disease. There were no complications, and the TEVG was patent on angiography seven months postoperatively. [14]
This method was repeated on two additional patients. Although it was successful, the culture and seeding process had several drawbacks. The biopsy forced patients to undergo an additional procedure on a date prior to their planned surgery. In addition, the cell culture process was labor-intensive and time-consuming, requiring 6–10 weeks to generate an adequate number of cells. As a result, there was an associated infectious risk and even the potential for malignant dedifferentiation of the cells during in vitro culture. [24] Perhaps most significant was the unreliability of this method, as we were unable to culture a sufficient number of cells in some patients.
As a result, we began investigating other potential sources of cells. Noishiki et al. had reported that bone marrow cells (BMCs) implanted onto the surface of an expanded polytetrafluoroethylene (ePTFE) graft led to endothelialization in a dog model. [25] In addition, bone marrow is known to contain multi-potential cells which have the ability to differentiate into several different cell types, and endothelial progenitor cells are derived from bone marrow. [26] Consequently, we investigated the utility of seeding biodegradable scaffolds with BMCs rather than cultured cells from a peripheral vein biopsy. BMCs were aspirated from the iliac bone of dogs and seeded onto a copolymer of lactic acid and ε-caprolactone. A neovessel composed of endothelial cells and smooth muscle cells formed, and the grafts remained patent, suggesting BMCs are a viable cell source. [27] The histologic composition of the neovessel was similar to grafts seeded with cultured venous cells, without the aforementioned shortcomings. [28,29] Unseeded grafts, in contrast, lacked a regular endothelial cell lining. [28] As we proceeded to use BMCs to seed synthetic grafts, we found that the mononuclear cell fraction of the harvested bone marrow is sufficient to generate a functional endothelium. [29] Moreover, enough cells could be harvested from a single procedure to enable adequate seeding without the need for ex vivo expansion, rendering bone marrow-derived mononuclear cells (BM-MNCs) ideal for seeding. The resultant neovessel demonstrated growth capacity, underlining its great potential in congenital cardiac surgery. [7]
Other groups have investigated the utility of genetic engineering of seeded cells to optimize tissue ingrowth and graft patency. Bone marrow mesenchymal stem cells have been genetically modified with endothelial nitric oxide synthase to increase the patency of small-diameter TEVGs. [30] Endothelial cells have also been genetically modified. Preis et al. recently induced expression of vascular endothelial growth factor165 (VEGF165) and fibulin-5 in endothelial cells and seeded them onto small-caliber ePTFE grafts. [31] Fibulin-5 is an extracellular matrix protein which participates in elastin fiber formation, and VEGF165 is an endothelial cell mitogen. Co-expression of VEGF165 and fibulin-5 in seeded endothelial cells significantly increased final histologic endothelial cell coverage and graft patency compared to seeding with naïve endothelial cells. [31] However, fibulin-5 inhibits smooth muscle cell migration, and it is unknown what effect this would have on neotissue formation in a tissue-engineered graft.
5. Clinical results of tissue-engineered vascular grafts
Given the success of TEVGs in animal models, we proceeded with the first clinical trial of TEVG implantation in children with congenital heart disease in Japan in 2001. [32,33] Our selected clinical target was the extracardiac Fontan procedure. As discussed above, other congenital cardiac operations have the potential to be impacted more significantly by the successful use of TEVGs, but the Fontan represented an optimal balance between utility and safety, with the conduits implanted in a high-flow, low-pressure circulatory system.
Hybrid tubular scaffolds composed of woven PGA and ε-caprolactone (50:50 ratio) and L-lactide were seeded with BM-MNCs. BM-MNCs were collected by density centrifugation with Histopaque-1077 (Sigma Chemical Co., St. Louis, MO, USA) after aspiration of 5 mL/kg of bone marrow from the anterior superior iliac spine. [32] The diameter and length of the scaffold were determined by each patient’s anatomy.
Patients younger than 30 years with minimal extracardiac disease burden presenting for elective surgery were included. Between 2001 and 2004, 25 patients underwent an extracardiac total cavopulmonary connection with a TEVG used as a conduit (Table 2). The median patient age at implantation was 4 years with a mean weight of 19.5 kg. Anticoagulation with warfarin and aspirin was started two days postoperatively and continued for 3–6 months. Patients were followed postoperatively in a multidisciplinary clinic and monitored radiographically with transthoracic echocardiography, computed tomography, angiography, or MRI angiography. Serial imaging demonstrated long-term growth capacity of the grafts (Figure 2). Upon autopsy, their gross and histologic appearance was similar to native vasculature (Figure 3).
Table 2.
Patient status after TEVG implantation. All grafts are patent, but seven (28%) were complicated by stenosis. PLA, polylactic acid; PGA, polyglycolic acid; PTA, percutaneous transluminal angioplasty.
| Patient ID # | Age at surgery (yrs) | Graft type | Graft diameter (mm) | Patient status | Graft-related complications | Number of PTA | Postoperative year of PTA |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2 | PLA | 16 | Alive | None | ||
| 2 | 1 | PLA | 20 | Alive | None | ||
| 3 | 8 | PLA | 18 | Dead | Stenosis | 1 | 5 |
| 4 | 22 | PLA | 24 | Alive | None | ||
| 5 | 13 | PLA | 22 | Dead | None | ||
| 6 | 4 | PLA | 20 | Alive | Stenosis | 1 | 8 |
| 7 | 14 | PLA | 24 | Dead | None | ||
| 8 | 17 | PLA | 24 | Alive | None | ||
| 9 | 22 | PLA | 22 | Dead | None | ||
| 10 | 4 | PLA | 12 | Dead | Stenosis | 3 | 5 |
| 11 | 2 | PLA | 16 | Dead | None | ||
| 12 | 2 | PGA | 16 | Alive | Stenosis | 4 (stent) | 1 |
| 13 | 2 | PGA | 16 | Alive | Thrombosis, stenosis | 1 | 12 |
| 14 | 2 | PGA | 18 | Alive | None | ||
| 15 | 2 | PGA | 12 | Alive | Stenosis | ||
| 16 | 2 | PGA | 16 | Dead | None | ||
| 17 | 24 | PGA | 18 | Alive | None | ||
| 18 | 1 | PGA | 16 | Alive | Stenosis | 1 | 5 |
| 19 | 11 | PGA | 18 | Alive | None | ||
| 20 | 2 | PGA | 14 | Alive | None | ||
| 21 | 3 | PGA | 16 | Alive | None | ||
| 22 | 5 | PGA | 18 | Alive | None | ||
| 23 | 4 | PGA | 18 | Alive | None | ||
| 24 | 13 | PGA | 16 | Alive | None | ||
| 25 | 2 | PGA | 18 | Dead | None | ||
Figure 2.
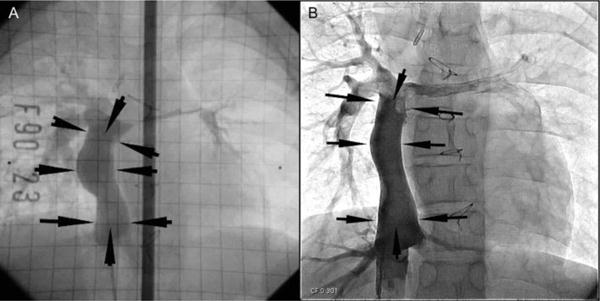
Postoperative growth of a TEVG. A TEVG was implanted in a five-year-old patient undergoing a Fontan procedure. Angiography two years (A) and eleven years (B) after implantation demonstrate growth, with length of the graft increased from 43.4 cm to 60.4 cm.
Figure 3.
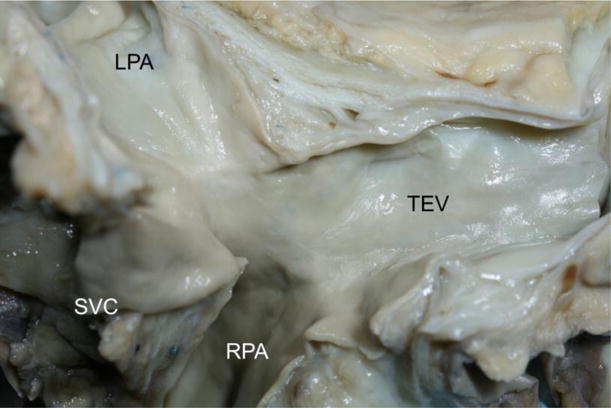
Gross image of a TEVG 13 years after implantation. The appearance is similar to native vein. LPA, left pulmonary artery; RPA, right pulmonary artery; SVC, superior vena cava; TEV, tissue-engineered vessel.
At 30 days post-implantation, all patients were alive and symptom-free, and all grafts were patent without stenosis, thrombosis, or aneurysmal dilation. By one year post-implantation, partial mural thrombosis was identified in one patient, which was successfully treated with warfarin. One patient with hypoplastic left heart syndrome died of congestive heart failure secondary to severe tricuspid regurgitation six months after TEVG implantation. [32]
With longer-term follow up, the most prevalent complication was graft stenosis. At a mean follow up of 5.8 years, four patients (16%) had stenosis on routine surveillance imaging. [33] The prevalence increased to seven patients (28%) at the most recent follow up (mean of 10.3 years, unpublished data). In all cases, stenosis has been treated successfully with balloon angioplasty, with one patient requiring stent placement in the stenotic segment of the TEVG. A total of eight mortalities (32%) have occurred since implantation, none of which have been related to the TEVG. There have been no instances of aneurysm formation, graft rupture, or ectopic calcification. [32,33]
In 2011, we began the first US Food and Drug Administration-approved clinical trial in the United States investigating TEVG use in children with congenital heart defects. The study is ongoing, but similarly to the Japanese cohort, the most common complication to date has been graft stenosis (unpublished data).
6. Cellular signaling and the important role of seeded cells
After the initial clinical study in Japan, TEVGs were taken back to animal models to develop strategies to reduce the incidence of postoperative stenosis. A murine model of TEVG implantation has enabled us to take advantage of the power of mouse genetic models. Because there are no animal models of single ventricle cardiac anomalies, we developed an inferior vena cava (IVC) interposition graft model as a surrogate, in which a segment of mouse IVC is replaced with a biodegradable scaffold 0.8 mm in diameter by 3 mm in length. [34] Although there are hemodynamic differences between the IVC interposition model and the postoperative circulation in patients with single ventricle anomalies, the model accurately replicates many aspects of the process of neovessel formation in large animals and humans, but over a shorter time course. [34–36]
We have performed several mechanistic studies that have helped elucidate the molecular mechanisms underlying neotissue formation. Cell seeding is not essential for neovessel formation, but it reduces the incidence of stenosis in TEVGs. [20,36,37] It does so in a dose-dependent manner, with greater numbers of seeded BM-MNCs significantly reducing the rate of stenosis. [20] The initial hypothesis was that BM-MNCs act as stem cells, differentiating into functional neotissue. Interestingly, the seeded cells disappear from the TEVG within a few days of implantation, suggesting that they are not the ultimate source of vascular neotissue and rather act via a paracrine mechanism. [37] Instead, the final neovessel cells are derived from the neighboring blood vessel. [35] BM-MNCs decline to 0.02% at 14 days, while the adjacent vessel wall forms 93% of the proximal neotissue. [35] Thus, seeded BM-MNCs appear to induce the host immune system to regenerate neotissue from pre-existing committed cells.
This discovery challenged the classical tissue engineering paradigm that stem cells differentiate into the final neotissue and shifted our focus toward investigating the host immune response to the TEVG. We subsequently found that macrophages play a critical role in neovessel formation. Host-derived monocytes infiltrate the scaffold as soon as three days after implantation. [36] Monocyte infiltration is crucial to the formation of the neovessel: blocking it prevents neotissue formation, while excessive infiltration results in stenosis. [36] Macrophage phenotype (M1 or M2) may also influence long-term TEVG function. [36] In short, achieving the correct balance of monocyte infiltration promotes successful neovessel formation. We have recently found that this process is mediated by the cytokine transforming growth factor beta (TGF-β). TGF-β signaling increases during neovessel formation, with stenotic grafts expressing higher levels of TGF-β than patent grafts. [38] A 14-day postoperative course of intraperitoneal TGF-β receptor 1 (TGF-βR1) inhibitor significantly reduces TEVG stenosis compared to unseeded controls, with patency rates in the unseeded TGF-βR1 inhibitor group similar to seeded grafts. [38] TGF-βR1 inhibitor significantly reduces activation of monocytes and macrophages, suggesting that it improves patency—without blocking neotissue formation—by reducing host monocyte activation. [38] There is still much to learn regarding the role played by seeded cells, but it is clear that the BM-MNCs themselves are not the source of neotissue, instead acting in a paracrine fashion to modulate the recipient’s immune response.
7. The future of vascular tissue engineering
Future directions will include the application of these mechanistic discoveries to design an improved TEVG. The immune response may be targeted directly with TGF-β inhibitors or, in the more immediate future, losartan, which also acts on the TGF-β pathway. Given the direct correlation between number of seeded cells and graft patency, we are investigating methods to increase the number of cells seeded onto the scaffold. One such method is the aspiration of a greater volume of bone marrow. We are investigating the feasibility of a larger bone marrow aspiration hours prior to major cardiac surgery, and at what cell dose the scaffold becomes saturated. As a corollary to this aim, a more efficient method of separating the mononuclear cell fraction would be useful. Layer separation by Histopaque is time-consuming, and the time during which the patient is under general anesthesia would be increased further by a large bone marrow aspiration. To that end, we developed a closed disposable system which is significantly faster than layer separation by Histopaque and also reduces the risk of contamination. [39]
Scaffold design will also continue to be refined, both to optimize tissue ingrowth and to customize the shape and length for each patient’s anatomy. Scaffold materials, fiber size and porosity will be adjusted to refine the mechanical properties and tissue ingrowth and potentially reduce the need for cell seeding. Three-dimensional printing is an emerging technology which offers exciting possibilities in scaffold construction. We have 3D printed TEVG scaffolds for implantation in mice and sheep. In the ovine model, electrospinning mandrels were 3D printed based on preoperative imaging, and TEVG scaffolds were then electrospun around the mandrels. [40] These TEVGs demonstrated satisfactory remodeling at six months postoperatively, with histology and mechanical properties similar to the native IVC. There is still work to be done before this technology is ready for translation to the clinic, but we anticipate a future in which a patient’s preoperative imaging will be used to design and print a unique scaffold for his or her surgical reconstruction.
8. Conclusion
Tissue-engineered vascular grafts can be implanted successfully in small and large animals, as well as humans, without significant dilation, thromboembolism, or infectious complications. The most common morbidity is graft stenosis, which can be successfully treated with balloon angioplasty. Results from murine studies indicate the critical role played by the seeded bone marrow-derived mononuclear cells. They reduce graft stenosis in a dose-dependent manner, suggesting that increasing the number of seeded cells in human TEVGs may reduce the incidence of stenosis. Additional investigation into the cellular signaling pathways mediating neotissue formation would further inform the development of an improved, second-generation TEVG.
In conclusion, TEVGs have found a successful clinical application in the field of congenital cardiac surgery. TEVG implantation in patients is promising, but there remains room for improvement. Research in small and large animal models has informed the mechanisms underlying neotissue formation and demonstrated the important role played by seeded cells, which act to modulate the recipient immune response. Further study in this exciting field will allow for optimization of neotissue formation and will broaden the clinical opportunities for TEVG use.
Acknowledgments
Dr. Drews has received grant support from Award Number Grant TL1TR001069 from the National Center For Advancing Translational Sciences.
Dr. Shinoka has received grant support from Gunze Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
References
- 1.van der Linde D, Konings EEM, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJM, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011 Nov;58(21):15. 2241–7. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Simeone RM, Oster ME, Cassell CH, Armour BS, Gray DT, Honein MA. Pediatric inpatient hospital resource use for congenital heart defects. Birth Defects Res Part A - Clin Mol Teratol. 2014;100(12):934–43. doi: 10.1002/bdra.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolk H, Loane M, Garne E. Congenital heart defects in Europe: Prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123(8):841–9. doi: 10.1161/CIRCULATIONAHA.110.958405. [DOI] [PubMed] [Google Scholar]
- 4.Giannico S, Hammad F, Amodeo A, Michielon G, Drago F, Turchetta A, et al. Clinical outcome of 193 extracardiac Fontan patients: the first 15 years. J Am Coll Cardiol. 2006;47(10):2065–73. doi: 10.1016/j.jacc.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 5.Homann M, Haehnel JC, Mendler N, Paek SU, Holper K, Meisner H, et al. Reconstruction of the RVOT with valved biological conduits: 25 years experience with allografts and xenografts. Eur J Cardio-thoracic Surg. 2000;17(6):624–30. doi: 10.1016/s1010-7940(00)00414-0. [DOI] [PubMed] [Google Scholar]
- 6.Jonas R, Freed M, Mayer J, Castaneda A. Long-term follow-up of patients with synthetic right heart conduits. Circulation. 1985;72(3 Pt 2):II77–83. [PubMed] [Google Scholar]
- 7.Brennan MP, Dardik A, Hibino N, Roh JD, Nelson GN, Papademitris X, et al. Tissue engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg. 2008;248(3):370–7. doi: 10.1097/SLA.0b013e318184dcbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karamlou T, Kotani Y, Van Arsdell GA. Congenital Heart Disease. In: Brunicardi F, Andersen DK, Billiar TR, Dunn DL, Hunger JG, Matthews JB, et al., editors. Schwartz’s Principles of Surgery. 10th. New York: McGraw-Hill; 2015. [Google Scholar]
- 9.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240–8. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nerem RM, Schutte SC. The challenge of imitating nature. In: Lanza RT, Langer R, Vacanti JP, editors. Principles of Tissue Engineering. 4th. San Diego, CA: Academic Press; 2014. pp. 9–24. [Google Scholar]
- 11.Dahl SLM, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011 Feb 2;3(68):68ra9–68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 12.Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothoracic Surg. 2003;23(6):1002–6. doi: 10.1016/s1010-7940(03)00094-0. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: State of the art. Tissue Eng Part B Rev. 2008 Mar;14(1):61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 14.Shin’oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344(7):532–3. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 15.Kumar V, Abbas AK, Fausto N. Robbins & Cotran Pathologic Basis of Disease. 7th. Philadelphia: Elsevier; 2004. p. 1525. [Google Scholar]
- 16.Walles T, Görler H, Puschmann C, Mertsching H. Functional neointima characterization of vascular prostheses in human. Ann Thorac Surg. 2004;77(3):864–8. doi: 10.1016/j.athoracsur.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 17.Naito Y, Shinoka T, Duncan D, Hibino N, Solomon D, Cleary M, et al. Vascular tissue engineering: Towards the next generation vascular grafts. Adv Drug Deliv Rev. 2011;63(4):312–23. doi: 10.1016/j.addr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Rosenman JE, Kempczinski RF, Pearce WH, Silberstein EB. Kinetics of endothelial cell seeding. J Vasc Surg. 1985 Nov;2(6):778–84. [PubMed] [Google Scholar]
- 19.Zilla P, Deutsch M, Meinhart J, Puschmann R, Eberl T, Minar E, et al. Clinical in vitro endothelialization of femoropopliteal bypass grafts: an actuarial follow-up over three years. J Vasc Surg. 1994 Mar;19(3):540–8. doi: 10.1016/s0741-5214(94)70083-4. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y-U, Mahler N, Best CA, Tara S, Sugiura T, Lee AY, et al. Rational design of an improved tissue-engineered vascular graft: determining the optimal cell dose and incubation time. Regen Med. 2016 Mar;11(2):159–67. doi: 10.2217/rme.15.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villalona GA, Udelsman B, Duncan DR, McGillicuddy E, Sawh-Martinez RF, Hibino N, et al. Cell-seeding techniques in vascular tissue engineering. Tissue Eng Part B Rev. 2010;16(3):341–50. doi: 10.1089/ten.teb.2009.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinoka T, Shum-Tim D, Ma PX, Tanel RE, Isogai N, Langer R, et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg. 1998;115(3):536–46. doi: 10.1016/S0022-5223(98)70315-0. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe M, Shin’oka T, Tohyama S, Hibino N, Konuma T, Matsumura G, et al. Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng. 2001;7(4):429–39. doi: 10.1089/10763270152436481. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura G, Hibino N, Ikada Y, Kurosawa H, Shin’oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24(13):2303–8. doi: 10.1016/s0142-9612(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 25.Noishiki Y, Tomizawa Y, Yamane Y, Matsumoto A. Autocrine angiogenic vascular prosthesis with bone marrow transplantation. Nat Med. 1996;2(1):90–3. doi: 10.1038/nm0196-90. [DOI] [PubMed] [Google Scholar]
- 26.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura G, Miyagawa-Tomita S, Shin’oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108(14):1729–34. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 28.Hibino N, Shin’oka T, Matsumura G, Ikada Y, Kurosawa H. The tissue-engineered vascular graft using bone marrow without culture. J Thorac Cardiovasc Surg. 2005;129(5):1064–70. doi: 10.1016/j.jtcvs.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura G, Ishihara Y, Miyagawa-Tomita S, Ikada Y, Matsuda S, Kurosawa H, et al. Evaluation of tissue-engineered vascular autografts. Tissue Eng. 2006;12(11):3075–83. doi: 10.1089/ten.2006.12.3075. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Qi H, Wang H, Hu P, Ou L, Guo S, et al. Engineering of vascular grafts with genetically modified bone marrow mesenchymal stem cells on poly (propylene carbonate) graft. Artif Organs. 2006;30(12):898–905. doi: 10.1111/j.1525-1594.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 31.Preis M, Schneiderman J, Koren B, Ben-Yosef Y, Levin-Ashkenazi D, Shapiro S, et al. Co-expression of Fibulin-5 and VEGF165 increases long-term patency of synthetic vascular grafts seeded with autologous endothelial cells. Gene Ther. 2016;23:237–46. doi: 10.1038/gt.2015.104. [DOI] [PubMed] [Google Scholar]
- 32.Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129(6):1330–8. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 33.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139(2):431–436.e2. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 34.Roh JD, Nelson GN, Brennan MP, Mirensky TL, Yi T, Hazlett T, et al. Small-diameter biodegradable scaffolds for functional vascular engineering in the mouse model. Biomaterials. 2008;29(10):1454–63. doi: 10.1016/j.biomaterials.2007.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hibino N, Villalona G, Pietris N, Duncan DR, Schoffner A, Roh JD, et al. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. FASEB J. 2011;25(8):2731–9. doi: 10.1096/fj.11-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hibino N, Yi T, Duncan DR, Rathore A, Dean E, Naito Y, et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J. 2011;25(12):4253–63. doi: 10.1096/fj.11-186585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107(10):4669–74. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee Y, Ruiz-Rosado J, Mahler N, Best CA, Tara S, Yi T, et al. TGF-b receptor 1 inhibition prevents stenosis of tissue-engineered vascular grafts by reducing host mononuclear phagocyte activation. FASEB J. 2016;30(7):2627–36. doi: 10.1096/fj.201500179R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hibino N, Nalbandian A, Devine L, Martinez RS, McGillicuddy E, Yi T, et al. Comparison of Human Bone Marrow Mononuclear Cell Isolation Methods for Creating Tissue-Engineered Vascular Grafts: Novel Filter System Versus Traditional Density Centrifugation Method. Tissue Eng Part C Methods. 2011;17(10):993–8. doi: 10.1089/ten.tec.2011.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukunishi T, Best CA, Sugiura T, Opfermann J, Ong CS, Shinoka T, et al. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. J Thorac Cardiovasc Surg. 2017;153(4):924–32. doi: 10.1016/j.jtcvs.2016.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]


