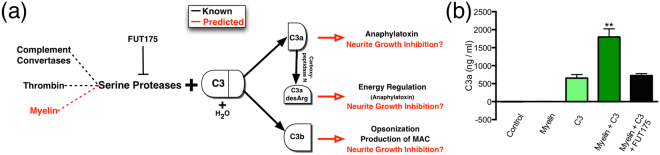
Figure 4.
Complement C3 can be cleaved by a serine protease in myelin. (a) Diagram of C3 cleavage including tested hypotheses. Complement convertases are known serine proteases that cleave C3 into C3a and C3b, which are functionally active immune proteins; in turn, C3a is converted to C3a desArg by carboxypeptidase N. FUT-175 inhibits serine protease activity and prevents C3 cleavage. Spontaneous hydrolysis of C3 also occurs at a relatively low level generating C3bH20. We predicted (in red) that serine proteases in myelin could cleave C3, and that newly formed active cleavage products (C3b, C3a, C3a desArg) may mediate neurite outgrowth inhibition and/or neuron death. These hypotheses are tested in Figs 4b and 5. (b) Results of C3a ELISA following incubation of C3 alone, C3+ myelin, and C3+ myelin + FUT-175. As expected, essentially no C3a was detected in control or myelin wells, and a small amount of C3a was detected with C3 alone. However, myelin significantly increased the generation of C3a, and this effect was blocked by FUT-175, indicating that serine proteases in myelin are capable of C3 cleavage. Mean ± SEM. ***p < 0.001 ANOVA, with **p < 0.01 Dunnett’s post-hoc t-test vs. C3 alone. N = 2 biological replicates for per group.