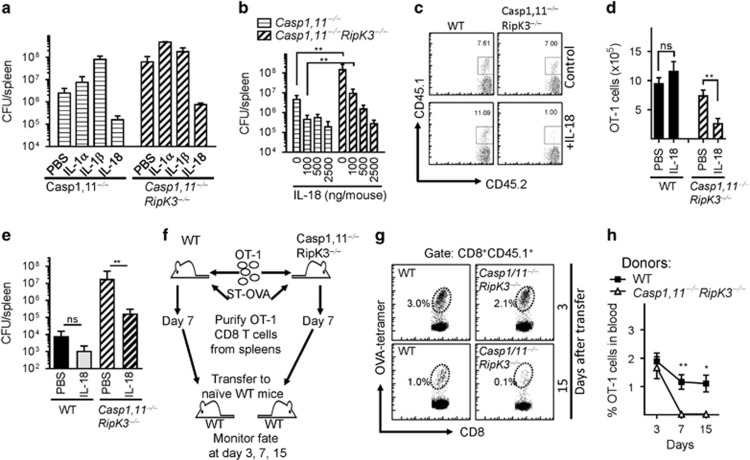
Figure 8.
Absence of Caspase-1,11 and RipK3 signaling compromises the survival of primed CD8+ T cells in a cell extrinsic manner. (a) Mouse recombinant IL-1α (100 ng per mouse.), IL-1β (100 ng per mouse) and IL-18 (1000 ng per mouse) was administered to Caspase-1,11−/minus; and Caspase-1,11−/minus;RipK3−/minus; mice (i.p.) from day -1 to 5 post-infection with ST-OVA (103 i.v.). Spleens were obtained from infected mice on day 6 post-infection, and the bacterial burden evaluated. (b) Different doses of IL-18 were injected to infected Caspase-1,11−/minus; and Caspase-1,11−/minus;RipK3−/minus; mice from day 1 to 5, and bacterial burden evaluated at day 6 post-infection. (c–e) OT-1 CD8+ T cells (CD45.1+CD45.2+) were injected (104, i.v.) into WT and Caspase-1,11–RipK3-double-deficient mice. Mice were infected with ST-OVA as described above. A group of mice received IL-18 (1000 ng per mouse) as indicated above. At day 7 post-infection, the numbers of donor OT-1 (CD45.1+CD45.2+) (c,d) and bacterial burden (e) were evaluated in the spleens of infected recipient (CD45.1−CD45.2+) mice. (f–h) OT-1 cells (CD45.1+CD45.2+) were transferred into WT and Caspase-1,11–RipK3-double-deficient mice, which were then infected with ST-OVA as described above. At day 7 post-infection, spleens were removed from infected mice, and the OVA-specific CD8+ T cells purified by staining with OVA-tetramers, biotin anti-CD45.1 followed by isolation with streptavidin magnetic beads. Purified cells from WT and Caspase-1,11–RipK3-double-deficient mice were then transferred into naive WT mice and the numbers of these cells evaluated in the blood at various time intervals post-transfer. The data shown are representative of 2-3 separate experiments. Statistical significance was determined by unpaired student t-test (*P<0.05, **P<0.01)