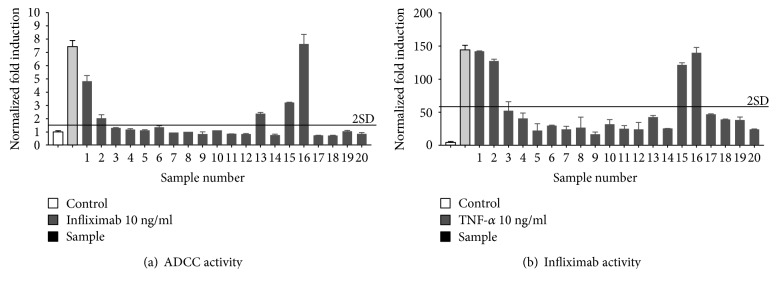
Figure 18.
Quantification of the ADCC activity of infliximab in the sera of patients with Crohn's disease. iLite effector cells (1.2 × 105 cells/well) were mixed with mTNFα target cells at an E:T ratio of 3 : 1 for 6 hours alone or in the presence of 10 ng/ml of infliximab or in the presence of an individual serum sample at a final dilution of 1/20 prior to the addition of Nano-Glo Dual-luciferase reagent (Promega, Madison, WI) and the sequential determination of FL and NL activity. Results are expressed as normalized fold ADCC activity relative to the control sample without infliximab (a). The same serum samples were analyzed for residual infliximab using an ELISA (Matriks Bioteck, Germany) or anti-TNFα activity in the presence of 10 ng/ml of TNFα using a reporter gene assay as described previously [23]. Results as expressed as normalized fold infliximab activity (b).