Abstract
Introduction:
High relapse rate of nonmuscle invasive bladder cancer (NMIBC) is a major challenge. Overexpression of microRNA-21 (miR-21) which targets phosphatase and tensin homolog (PTEN), a gene associated with malignancy, has been reported in the bladder tumor tissue compared to normal mucosa by us and others. We have tested whether miR-21 levels in bladder mucosa could predict tumor recurrence.
Methods:
In a prospective cohort setting, tumor tissues and normal bladder mucosa (NBM) were taken from BC patients during transurethral resection of bladder tumor. Age- and ethnicity-matched NBM from benign prostate hyperplasia patients was taken as controls. The expression of miR-21 was analyzed using quantitative reverse transcription polymerase chain reaction. Patients were followed for 4 years for tumor reoccurrence. Postoperative recurrence were recorded and calculated by Kaplan–Meier curve.
Results:
In 31 patients, miR-21 was up-regulated (>4-fold, P = 0.003), and PTEN levels were significantly lower (<7-folds, P = 0.001) in tumor tissue relative to NBM. Moreover, the fold change in miR-21 levels was significantly higher (>3-folds, P = 0.03) in patients showing recurrence compared to those in which tumor did not recur. Further, Kaplan–Meier analysis shows overexpression of miR-21 corresponds to less time to recurrence with higher cumulative hazard.
Conclusion:
We found overexpression of miR-21 in tumor tissue and its association with recurrence, time to recurrence and invasiveness in BC. Quantification of miR-21 along with other pathological parameters could be more objective molecular approach to predict recurrence in NMIBC.
INTRODUCTION
Bladder cancer (BC) is the fifth most common cancer in men in India with an incidence rate of 5.8/100,000 person years.[1] Pathological characterization of BC including stage and grade of tumor are in current use to predict the disease recurrence. However, due to the non-absolute nature of existing pathological characteristics, lot many patients of nonmuscle invasive BC (NMIBC), a more common form of BC, have to undergo painful invasive cystoscopic examination on a regular interval.[2,3] In addition, it poses a dilemma in decision-making particularly for T1 high grade.[4] Thus, there is a need of molecular marker for absolute characterization of tumors beyond the existing pathological parameters. Such markers could clearly suggest a probability of recurrences or progression and will help to prognosticate the outcome of initial treatment in patients with NMIBC.
MicroRNAs (miRs) are short (20–24 nucleotides) non-coding RNA, mostly transcribed from intronic regions of genomic DNA and functions as negative regulators of gene expression by RNA interference.[5,6,7] MiR expression studied on wide spectrum of cancer diseases has explained its potential as oncogene, tumor suppressor gene, and epigenetic regulator.[8,9,10,11] Of various miRs, miR-21, which mostly governs the pathway of epithelial to mesenchymal transition (EMT), has been shown to be overexpressed in bladder tumor as compared to the normal mucosa by us and others.[12,13,14] Moreover, phosphatase and tensin homolog (PTEN) is an experimentally validated target (miRTar Base Database) of miR-21, which is a tumor suppressor gene, known to be down-regulated by miR-21 in many cancers.[15,16,17]
Despite an association of miR-21 with several cancers its relationship with tumor recurrence in BC patients, especially NMIBC, is far from clear.[18,19] In our previous study,[14] we found miR-21, miR-205, miR-126, miR-10b, and miR-200a to be highly expressed in tumor tissue of BC patients out of nine selective miRs previously reported to be dysregulated in different cancers. In which expression of miR-21 and miR-129 was correlated with clinical parameters of BC. This study was designed to determine if miR-21 could be used to prognosticate the outcome of initial treatment in patients with NMIBC in a more absolute way. For this, we analyzed expression of miR-21 and its target gene in tumor and normal bladder mucosa (NBM) tissues from BC patients between October 2011 and September 2015. NBM taken from benign prostate hyperplasia (BPH) patients of similar age and ethnicity were taken as control (C). MiR-21 expressions were seen in relation with recurrence and time to recurrence to determine its usefulness as prognostication marker.
METHODS
Study subject enrollment
This study was approved by Institutional Ethics Committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow (A-02:PGI/IMP/IEC/57/21.10.2011). Forty patients with primary BC were enrolled, and their tumor and NBM sample were taken for this single center study. Recruitments were done between October 2011 and September 2015 after taking informed consents. Out of forty BC patients, nine BC patients were excluded as they had muscle invasive tumors. Twelve NBM as healthy control (HC) were also obtained from patients of similar age group without cancer and having transurethral surgery for BPH. All the tumor tissues from BC patients were taken from en bloc resected tumors. Tumors were visually identified in bladder lumen, which were further confirmed by histopathology. Demographic data [Table 1] were captured using a standard clinical pro forma. Smoking could be a significant confounding factor and number of smokers was higher in the study group (10/31) than the control group (2/12), but due to a small number of patients, we did not do multivariate analysis. Follow-up cystoscopies were done at a regular interval to see for recurrence. Patients were not given intravesical Mitomycin C immediately after TURBT and Bacillus–Calmette–Guerin (BCG) was given as per the protocol of the department whenever indicated, i.e. for the recurrent low-grade, high-grade lamina invasive tumor, etc., BCG was given in the doses of 80 mg for 6 weekly doses and was repeated in case of recurrence without maintenance. Tissue samples (~200 mg) from patients and controls were collected in RNA later and stored at −80°C until RNA extraction.
Table 1.
Demographic details and pathological tumor characteristics of the patients

Fold change in miR-21 level was normalized to expression of miR-21 in NBM of BPH patients and correlated with different clinical parameters of patients.
As there were only 31 samples, so cutoff level (10-fold) was decided conventionally by looking at the data and fold change of miR-21 were pooled into two Groups 1 and 2, i.e., ≤10 and >10, respectively, for Kaplan–Meier analysis [Table 1].
Histopathological examinations
All tissue samples were immediately fixed in 10% buffered formalin and embedded in paraffin wax for histopathology. Formalin fixed paraffin embedded tissue sections were cut at a thickness of 5–6 μm using a microtome followed by staining with hematoxylin and eosin for pathological assessment by the Department of Pathology, SGPGIMS, Lucknow. An average of 2 − 5 sections was examined for each tissue sample for defining stages and grades of BC. Staging of tumors was done as per tumor-node-metastases classification defined by American Joint Committee on Cancer.[20] Tumors were classified in grades as per the guidelines of the World Health Organization/International Society of Urological Pathology.[21]
RNA isolation
RNA isolation from 50 to 100 mg tissue samples, were performed using mir-Vana miRNA isolation kit (Ambion, USA) according to the manufacturer's instructions. Concentration and purity were controlled using Nanodrop. Only RNA samples with 260/280 ratios of 1.8–2.0 were used for investigation.
Complementary DNA synthesis of microRNA
Complementary DNA (cDNA) was synthesized from RNA using miRNA-specific looped primers according to the TaqMan MicroRNA assay protocol (PE Applied Biosystems, Foster City, CA, USA). For reverse transcription (RT) reactions, 10 ng of RNA sample, 50 nM of stem-loop RT primer, 1X RT buffer and 0.25 mM each of dNTPs, 3.33 U/μL MultiScribe RT, and 0.25 U/μL RNase inhibitor (all from the TaqMan MicroRNA Reverse Transcription kit of Applied Biosystems; 4366597) were utilized. Reactions were incubated in thermocycler (BioRad, USA) at 16°C for 30 min, 42°C for 30 min, 85°C for 5 min, and 4°C for 10 min.
Quantification of microRNAs by TaqMan method
Quantitative polymerase chain reaction (qPCR) was performed using the Applied Biosystems 7800 Sequence Detection System. The 20-μL PCR reaction mixture included 1.3 μL of RT product, 1X TaqMan (No UNG) Universal PCR Master Mix and 1 μL of primer and probe mix of the TaqMan MicroRNA Assay protocol (PE Applied Biosystems, USA). Reactions were incubated in a 96-well optical plate at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 10 min. The threshold cycle (CT) data were determined using the default threshold settings. All reactions were run in triplicate and average CT and standard deviation (SD) values were calculated. Expression data were normalized according to the expression of the RNU-48 (endogenous control) (Applied Biosystem).
MicroRNA target prediction
Of 4 overexpressed miRNAs, i.e. miRNA 21, miRNA129, MiRNA 200a, and miRNA 205 in tumor tissue assessed in an earlier study done at our center,[14] miR-21 was significantly associated with the clinical outcome in NMIBC and was selected to find out the target mRNA for functional analysis. This was done using bioinformatics database Target Scan (7.0, http://www.targetscan.org) and experimentally validated target database miRTar Base. PTEN which is tumor suppressor gene was selected as target of miR-21. Fold change in PTEN level, target gene of miR-21 in tumor tissues of 13 patients was compared with normal mucosa of BC patients (both normalized with normal mucosa of HC, i.e., BPH patients).
First strand complementary DNA synthesis of mature microRNA
cDNA was synthesized from total RNA using oligo dT primers according to the Thermo Scientific RevertAid Premium First Strand cDNA Synthesis Kit (#K1652). Each cDNA synthesis reaction utilized 100 ng of RNA, 25 pmol (0.25 μL) oligo (dT)18 primer, 10 mM dNTP Mix (1 μL), 5X RT buffer (4 μL), RevertAid Premium Enzyme Mix (1 μL), nuclease free water (up to 20 μL). Reactions were incubated in thermocycler (BioRad) at 25°C for 10 min, 50°C for 15 min, 85°C for 5 min, and 4°C for 10 min.
Quantification of target mRNA by SYBR green method
qPCR was performed using the Applied Biosystems 7800 Sequence Detection System. The 10-μL PCR reaction mixture included 1 μL of first strand cDNA, 5 μL SYBR Premix Ex Taq (TaKaRa Clontech), 1 μL Forward Primer (1 pmol), 1 μL Reverse Primer (1 pmol), and 5 μL water. Reactions were incubated in a 96-well optical plate at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 10 min. The threshold cycle data were determined using the default threshold settings. All reactions were run in triplicate, and average CT and SD values were calculated. Expression data were normalized according to the expression of the GAPDH (endogenous control) (Applied Biosystem).
Primer sequences (Imperial Life Sciences (P) Limited)
PTEN Forward: CGAACTGGTGTAATGATATGT
PTEN Reverse: CATGAACTTGTCTTCCCGT
GAPDH Forward: CTTCACCACCATGGAGGAGGC
GAPDH Reverse: GGCATGGACTGTGGTCATGAG.
Immunohistochemistry of tumor tissues
miR-21 is involved in EMT pathway; a process of tumor invasion and metastasis. EMT is characterized by loss of E-cadherin and increased expression of Vimentin. Immunohistochemistry of formalin fixed paraffin embedded tissue was done using anti-Vimentin and anti-E-cadherin antibodies from a commercially available kit SS™ * IHC Detection System, (BioGenex, USA). Power block, pre-diluted ready to use primary antibody (RTU-1 Ab), Enhancer, polymer horseradish peroxidase-conjugated secondary antibody and diaminobenzidine substrate all were used by a kit SS™ * IHC Detection System, (BioGenex) according to manufacturer's protocol. ImageJ IHC profiler was used for IHC scoring of cytosolic Vimentin and trans-membranous E-cadherin.[22]
Western blot analysis
We evaluated the end product of the target gene, i.e., PTEN by measuring its protein level through western blot. Total protein was extracted from tissue lysate, and its concentration was estimated by the Bradford assay. Polyacrylamide gel electrophoresis was run with 50 μg protein and was transferred on polyvinylidene difluoride membrane (Millipore Corp, Bedford, MA, USA). Membranes were incubated for 1 h at 25°C with Tris-buffered saline with Tween (TBS-T; 20 mM Tris [pH 7.6], 100 μM NaCl, 0.1% Tween-20), and 5% skimmed milk on rocker. Membranes were incubated overnight with primary antibodies in TBS-T buffer containing 5% milk at 4°C. Then, they were incubated with appropriate secondary antibodies in TBS-T/5% milk for 1–2 h at 25°C. The protein bands were visualized by enhanced chemiluminescent detection kit (Millipore, Billerica, USA). The membranes were stripped (2× for 15 min at 55°C) in stripping buffer containing 100 mM 2-mercaptoethanol, 2% sodium dodecyl sulfate, and 62.5 mM Tris-HCl (pH 6.7) and reprobed for β-actin. All experiments were performed at least three times. Proteins visualized by western blot were quantitated by densitometric analysis and normalize with β-actin (endogenous control).
Statistical analysis
Statistical analysis was performed by Wilcoxon matched pair t-test, Mann–Whitney test, Spearman's correlation, and Kaplan–Meier analysis using SPSS16 (IBM Corporation, NY, USA), Microsoft Excel and Graph Pad Prism6 (trial version). Fold change in expression by RT-qPCR was calculated by 2−ΔΔCt method, where ΔΔCt (Tumor) = ΔCtTumor − Average ΔCtHC and ΔΔCt (Normal) = ΔCtNormal − Average ΔCtHC.[23] Fold changes were log-transformed for statistical analyses and representation. Fold change of miR-21 was pooled into two Groups 1 and 2, i.e. ≤10 and >10 respectively for Kaplan–Meier analysis.
RESULTS
Of total forty patients of primary BC included for the study, nine had muscle invasion, so were excluded from the analysis. Mean age of the NMIBC patients and HCs were similar. Most of the patients were of low grade (n = 17) (54.8%) and of Ta stage (n = 19) (61.3%) and rest were of high grade (n = 14) (45.2%) and T1 stage (n = 12) (38.7%). All the patients were followed for a median time of 18 (2–49) months for recurrence. Recurrence was observed in 22 patients (55%) with a median time to recurrence 7 (2–49) months [Table 1].
Higher expression of microRNA-21 in bladder tumor tissue compared to “normal” bladder mucosa from bladder cancer patients
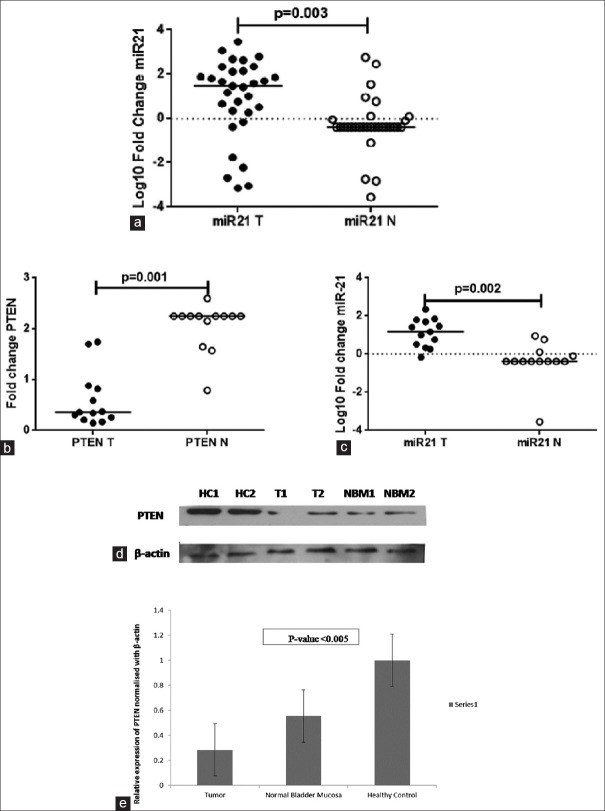
In agreement to our previous report, we found a significant higher fold change in miR-21 levels in tumor tissues with respect to the NBM from NMIBC patients. The median log10 fold changes were 1.5 (−3.1–3.5) and − 0.38 (−3.5–2.7), P = 0.003, respectively [Figure 1a].
Figure 1.
(a) Fold change of microRNA-21 in tumor and normal tissue (n = 31). (b) Fold change of phosphatase and tensin homolog and (c) microRNA-21 in respective samples (n = 13). (d and e) Expression of phosphatase and tensin homolog in tumor (t), healthy (HC), and normal (normal bladder mucosa) tissue
Association of microRNA-21 with phosphatase and tensin homolog
Moreover, median value of fold change in PTEN levels was significantly lower in tumor tissue (n = 13) relative to normal mucosa (n = 13) from BC patients. The median fold changes were, i.e. 0.36 (0.15–1.74) and 2.3 (0.8–2.3), respectively (P = 0.001) [Figure 1b]. Median value of fold change in miR-21 levels in respective tumor samples (n = 13) (for which PTEN expression was evaluated) was significantly (P = 0.002) lower in tumor tissue (n = 13) relative to normal mucosa (n = 13) from BC patients [Figure 1c].
The reduced mRNA levels of PTEN in tumor tissue were further confirmed by significant (P < 0.005) lower levels of PTEN protein in tumor tissue from NMIBC patients as compared to NBM and HC [Figure 1d and e].
Fold change in microRNA-21 is associated with tumor grade and invasiveness in bladder cancer patients
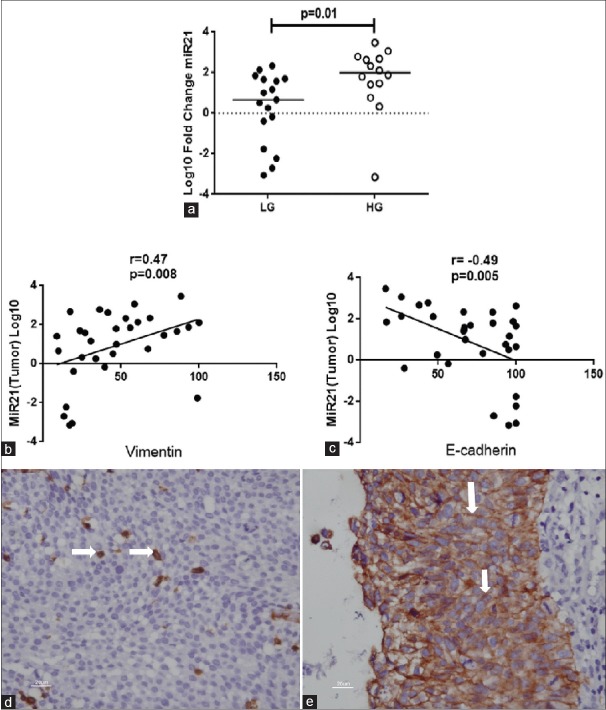
In high-grade tumors, the median fold change for miRNA-21 was significantly higher relative to low-grade of NMIBC. The median log10 fold changes were 2 (−3.1–3.5) and 0.66 (−3–2.35), P = 0.01, respectively [Figure 2a]. Moreover, fold change in miR-21 level positively correlated (correlation coefficient = 0.46; P = 0.009) with tumor grade C.
Figure 2.
(a) Fold change of microRNA-21 in high grade (n = 14) and low grade (n = 17) tumors. (b) Correlation of microRNA-21 with Vimentin, and (c) E-cadherin n = 31/group. (d and e) Immunohistochemistry showing expression of Vimentin and E-cadherin, respectively (white arrow)
In addition, it correlated positively (r = 0.47, P = 0.008) with IHC score of Vimentin and negatively (r = −0.49, P = 0.005) with IHC score of E-cadherin [Figure 2b–e].
Bladder cancer patients with relatively greater fold change in microRNA-21 showed recurrence
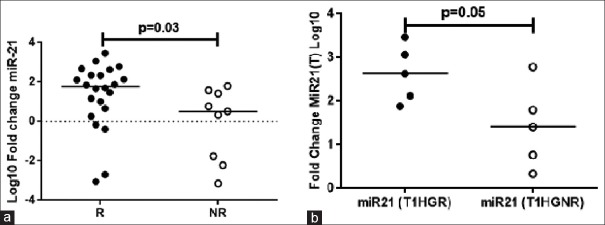
Median fold change of miR-21 in bladder tumor tissue was significantly (P = 0.03) higher in NMIBC patients showing recurrence in ~7 months compared to patients who did not show recurrence. The median log10 fold changes were 1.8 (−3.1–3.5, n = 22) and 0.5 (−3.1–1.8, n = 9), respectively [Figure 3a].
Figure 3.
(a) Fold change of microRNA-21 in recurrence (n = 22) and non-recurrence (n = 9) patients. (b) Fold change of microRNA-21 in lamina-invasive high-grade recurrence (T1HGR) and non-recurrence (T1HGNR) tumors (n = 5/group)
In addition, the high-grade lamina-invasive BC patients with a greater median log10 fold change in miR-21 had a recurrence in ~10 months (n = 5 out of 10). While recurrence did not occur in the high-grade lamina-invasive BC patients with relatively lower median fold change in miR-21. The median log10 fold changes were 2.6 (1.8–3.5) and 1.4 (0.33–2.7), P = 0.05, respectively [Figure 3b] (P = 0.05).
Higher expression of microRNA-21 in nonmuscle invasive bladder cancer corresponds to less time to recurrence with greater hazard
Fold change of miR-21 was grouped in lower and higher, i.e. 1 ≤10 and 2 >10 respectively for Kaplan–Meier time to recurrence analysis. Nineteen (61.3%) patients had >10-fold change of miR-21 in which 15 patients had recurrence in median time of ~10 months. Seven out of 12 patients having ≤10-fold change of miR-21 have recurrence in median time of ~30 months [Table 1]. A Kaplan–Meier curve was generated, which indicated that expression levels >10-fold were significantly related to recurrence (P = 0.05) which means increased expression of miR-21 promotes recurrence of NMIBC. Higher expression of miR-21 corresponds to less time to recurrence with higher cumulative hazard, having hazard ratio of 0.64 at 95% confidence interval (log rank = 0.03) [Figure 4].
Figure 4.
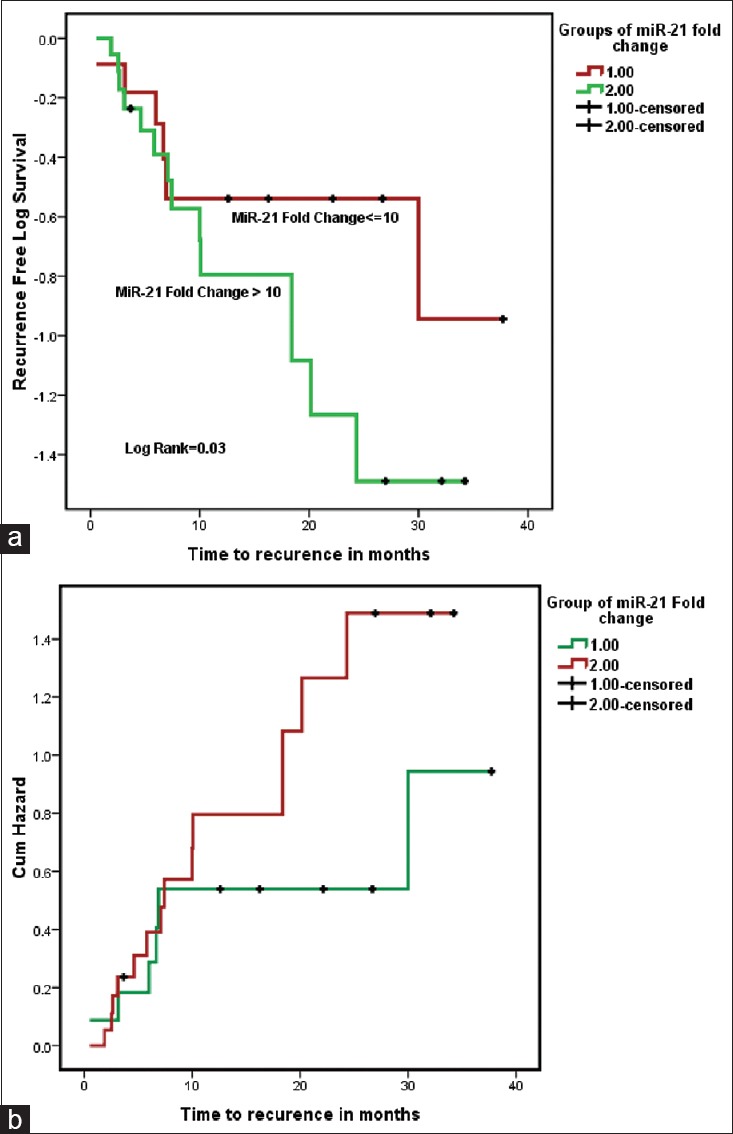
Higher expression of microRNA-21 (Group 2 = fold change >10) (n = 19) corresponds to less time to recurrence (a) with higher cumulative hazard (b), having hazard ratio of 0.64 at 95% confidence interval (log rank = 0.03)
DISCUSSION
Two different tumors of same stage and grade may behave differently and the existing pathological parameters to prognosticate the outcome in NMIBC are not robust enough to make the treatment choice, particularly in high-grade lamina invasive tumors. Moreover, the existing pathological factors such as stage and grade are subjected to inter- and intra-observer variability. Nowadays, regular expensive, invasive, and painful follow-up cystoscopies is the only option to predict the outcome of treatment. There is a need for more accurate and objective criterion to classify risk groups to offer better treatment choice and follow-up strategy.
MiRs play important role in development and differentiation process through posttranscription gene regulation.[24] There are evidences which suggests misguided miRs mediate gene regulation in oncogenesis.[25,26] We and others have reported miR-21 dysregulation in BC as well as several other cancers, in which Dyrskjot et. al., found miR-21 to be the most up-regulated out of 290 miR profiled in BC.[8,12,14,27,28] In the present study, we again confirmed these reports by showing overexpression of miR-21 in the bladder tumor tissues from patients with BC. The association of miR-21 with colorectal cancer and prostate cancer had been reported[18,19] but it is the first time that we are reporting the association of overexpressed miR-21 in tumor of BC patients with recurrence and time to recurrence. Moreover, among the BC patients, the participants with recurrences of T1 high grade tumor were found to have higher expression of miR-21 in their bladder tissue. However, due to limited number of BC patents in this subgroup, i.e., of T1 high grade tumor the results should be interpreted with caution.
Nevertheless, our study found an association of higher expression of miR-21 in bladder tissue with grade of tumors in BC patients. This observation is in concurrence with study by Catto et al., showing differential expression of miR-21 with pathological characteristics like stage and grade of BC.[13] In addition, our findings suggests a possible association of miR-21 overexpression with EMT. EMT of a cell is associated as a cause for tumor aggressiveness by aiding proliferation, invasion, and migration of tumor cell. Upregulation of Vimentin and down-regulation of E-cadherin are known hallmarks for EMT.[29] Thus, a strong association of miR-21 levels with Vimentin (positive correlation) and E-cadherin (negative association) expression in tumors tissues from BC patients suggest that overexpression of miR-21 is associated with tumor aggressiveness.
Together, the overexpression of miR-21 and its associations with grade, invasiveness, and recurrence suggest a pathogenic role of miR-21 in bladder tumor oncogenesis. This is further supported by significantly lower expression of PTEN, a predicted target of miR-21, in tumor tissue from BC. PTEN is a tumor suppressor gene that negatively regulates cell proliferation and survival and its decreased expression induces constitutive phosphorylation of focal adhesion kinase, which is involved in cell cycle progression, survival and migration in glioblastoma.[30] However, more direct functional studies are required to prove this pathologic role of miR-21.
CONCLUSION
miR-21 mRNA is overexpressed in bladder cancer tissue and is associated with tumor recurrence, time to recurrence and invasiveness. The short duration of follow up and small size of cohort limit the strength of our study.
Financial support and sponsorship
This study was supported by intramural grant of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India. Mitash N. was supported by a fellowship grant from Council of Scientific and Industrial Research, New Delhi, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study was supported by intramural grant of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India. Mitash N. was supported by a fellowship grant from Council of Scientific and Industrial Research, New Delhi, India.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Messing EM. Urothelial tumors of the bladder. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9th ed. Philadelphia, PA: Saunders Elsevier; 2007. pp. 2407–46. [Google Scholar]
- 3.Dinney CP, McConkey DJ, Millikan RE, Wu X, Bar-Eli M, Adam L, et al. Focus on bladder cancer. Cancer Cell. 2004;6:111–6. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Kwak C, Ku JH, Park JY, Lee E, Lee SE, Lee C. Initial tumor stage and grade as a predictive factor for recurrence in patients with stage T1 grade 3 bladder cancer. J Urol. 2004;171:149–52. doi: 10.1097/01.ju.0000099825.98542.a8. [DOI] [PubMed] [Google Scholar]
- 5.Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 6.Mansfield JH, Harfe BD, Nissen R, Obenauer J, Srineel J, Chaudhuri A, et al. MicroRNA-responsive ‘sensor’ transgenes uncover Hox-like and other developmentally regulated patterns of vertebrate microRNA expression. Nat Genet. 2004;36:1079–83. doi: 10.1038/ng1421. [DOI] [PubMed] [Google Scholar]
- 7.Seitz H, Youngson N, Lin SP, Dalbert S, Paulsen M, Bachellerie JP, et al. Imprinted microRNA genes transcribed antisense to a reciprocally imprinted retrotransposon-like gene. Nat Genet. 2003;34:261–2. doi: 10.1038/ng1171. [DOI] [PubMed] [Google Scholar]
- 8.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–14. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munker R, Calin GA. MicroRNA profiling in cancer. Clin Sci (Lond) 2011;121:141–58. doi: 10.1042/CS20110005. [DOI] [PubMed] [Google Scholar]
- 11.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 12.Dyrskjøt L, Ostenfeld MS, Bramsen JB, Silahtaroglu AN, Lamy P, Ramanathan R, et al. Genomic profiling of microRNAs in bladder cancer: miR-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009;69:4851–60. doi: 10.1158/0008-5472.CAN-08-4043. [DOI] [PubMed] [Google Scholar]
- 13.Catto JW, Miah S, Owen HC, Bryant H, Myers K, Dudziec E, et al. Distinct microRNA alterations characterize high- and low-grade bladder cancer. Cancer Res. 2009;69:8472–81. doi: 10.1158/0008-5472.CAN-09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitash N, Agnihotri S, Mittal B, Tiwari S, Mandhani A. Molecular cystoscopy: Micro-RNAs could be a marker for identifying genotypic changes for transitional cell carcinoma of the urinary bladder. Indian J Urol. 2016;32:149–53. doi: 10.4103/0970-1591.174775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–29. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 16.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–58. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Mattos-Arruda L, Bottai G, Nuciforo PG, Di Tommaso L, Giovannetti E, Peg V, et al. MicroRNA-21 links epithelial-to-mesenchymal transition and inflammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in HER2-positive breast cancer patients. Oncotarget. 2015;6:37269–80. doi: 10.18632/oncotarget.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bullock MD, Pickard K, Mitter R, Sayan AE, Primrose JN, Ivan C, et al. Stratifying risk of recurrence in stage II colorectal cancer using deregulated stromal and epithelial microRNAs. Oncotarget. 2015;6:7262–79. doi: 10.18632/oncotarget.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leite KR, Reis ST, Viana N, Morais DR, Moura CM, Silva IA, et al. Controlling RECK miR21 promotes tumor cell invasion and is related to biochemical recurrence in prostate cancer. J Cancer. 2015;6:292–301. doi: 10.7150/jca.11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobin DH, Wittekind CH, editors. TNM Classification of Malignant Tumours.6th ed. 6th ed. New York: Wiley Liss; 2002. pp. 199–202. [Google Scholar]
- 21.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–48. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9:e96801. doi: 10.1371/journal.pone.0096801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich EM, Dimmeler S. MicroRNAs and stem cells: Control of pluripotency, reprogramming, and lineage commitment. Circ Res. 2012;110:1014–22. doi: 10.1161/CIRCRESAHA.111.243394. [DOI] [PubMed] [Google Scholar]
- 25.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–7. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 26.Peter ME. Let-7 and miR-200 microRNAs: Guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–52. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Fan Z, Liu F, Zuo J. Hsa-miR-21 and Hsa-miR-29 in tissue as potential diagnostic and prognostic biomarkers for gastric cancer. Cell Physiol Biochem. 2015;37:1454–62. doi: 10.1159/000438514. [DOI] [PubMed] [Google Scholar]
- 28.Winther M, Alsner J, Tramm T, Baeksgaard L, Holtved E, Nordsmark M. Evaluation of miR-21 and miR-375 as prognostic biomarkers in esophageal cancer. Acta Oncol. 2015;54:1582–91. doi: 10.3109/0284186X.2015.1064161. [DOI] [PubMed] [Google Scholar]
- 29.Morata-Tarifa C, Jiménez G, García MA, Entrena JM, Griñán-Lisón C, Aguilera M, et al. Low adherent cancer cell subpopulations are enriched in tumorigenic and metastatic epithelial-to-mesenchymal transition-induced cancer stem-like cells. Sci Rep. 2016;6:18772. doi: 10.1038/srep18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park MJ, Kim MS, Park IC, Kang HS, Yoo H, Park SH, et al. PTEN suppresses hyaluronic acid-induced matrix metalloproteinase-9 expression in U87MG glioblastoma cells through focal adhesion kinase dephosphorylation. Cancer Res. 2002;62:6318–22. [PubMed] [Google Scholar]