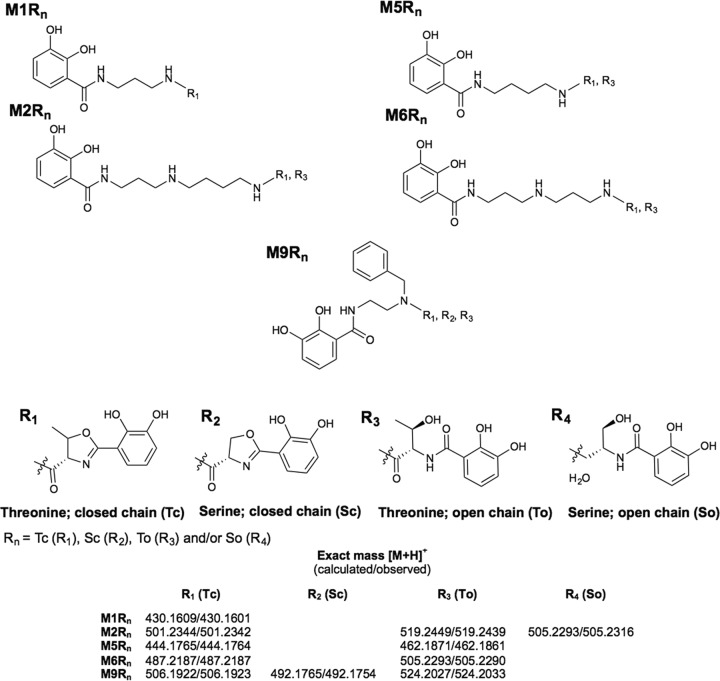
FIG 4 .
Proposed structures for the new serratiochelin analogs. The VibF acylation of the primary amine from the intermediates depicted in Fig. 3 can occur with 2-(2,3-dihydroxyphenyl)-5-methyloxazolinyl (R1 and R3) and/or a 2-(2,3-dihydroxyphenyl)-oxazolinyl (R2 and R4) as well. In some samples, the amino acid incorporated into the intermediate was found not to have gone through an additional cyclization, thus remaining in the open conformation as dihydroxybenzoyl-l-threonine and -serine (R3 and R4). Each molecule is identified by the letter “M” and a number, corresponding to the polyamine added to the medium, as well as the amino acid (S, serine; T, threonine) incorporated and its configuration (c, closed; o, open). Rn indicates the alternative radicals for the structures proposed and detected in the samples.