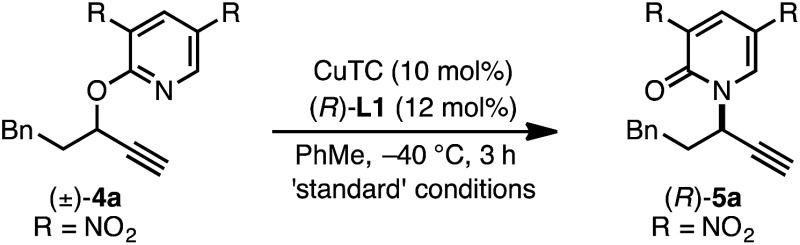
Table 1. Impact of reaction parameters on catalytic enantioconvergent formal [1,3]-rearrangement a .
| ||||
| Entry | Variation from ‘standard’ conditions | Conv. b (%) | Yield b (%) | er c |
| 1 | R = H | 0 | — | — |
| 2 | None | >98 | 90 | 97.5 : 2.5 |
| 3 | No CuTC | 0 | — | — |
| 4 | No (R)-L1 | 0 | — | — |
| 5 | CuOTf·0.5PhH, instead of CuTC | 66 | 50 | 77 : 23 |
| 6 | CuI, instead of CuTC | 88 | 50 | 47 : 53 |
| 7 | Cu(MeCN)4BF4, instead CuTC | 72 | 60 | 46 : 54 |
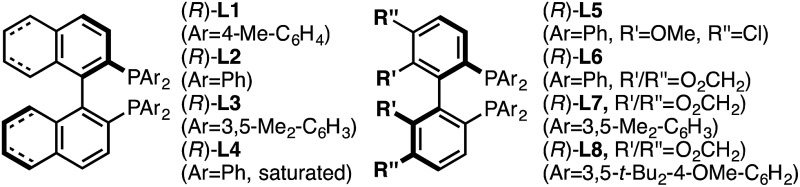
| 8 | (R)-L2, instead of (R)-L1 | >98 | 94 | 96.5 : 3.5 |
| 9 | (R)-L4, instead of (R)-L1 | >98 | 97 | 95.5 : 4.5 |
| 10 | (R)-L5, instead of (R)-L1 | >98 | 95 | 96.5 : 3.5 |
| 11 | (R)-L6, instead of (R)-L1 | >98 | 92 | 96.5 : 3.5 |
| 12 | (R)-L3, instead of (R)-L1 | 39 | 24 | 19 : 81 |
| 13 | (R)-L7, instead of (R)-L1 | 80 | 40 | 50 : 50 |
| 14 | (R)-L8, instead of (R)-L1 | <2 | — | — |
| 15 | THF, instead of PhMe | >98 | 80 | 96 : 4 |
| 16 | Addition of i-Pr2NEt (2.0 equiv.) | >98 | 82 | 96.5 : 3.5 |
| 17 | 5 mol% CuTC, 6 mol% (R)-L1 (7 h) | >98 | 85 | 97.5 : 2.5 |
| 18 | –20 °C (1 h), instead of –40 °C | >98 | 96 | 96 : 4 |
| 19 | 20 °C (10 min), instead of –40 °C | >98 | 87 | 94 : 6 |
| ||||
aAll data are the average of two experiments performed using 0.1 mmol substrate. For entries in which incomplete conversion was observed, reaction times were 24 h.
bDetermined by analysis of the crude reaction mixtures by 1H NMR using CH2Br2 as an internal standard.
cDetermined by chiral stationary phase HPLC. CuTC = copper(i)-thiophenecarboxylate. Bn = benzyl.
