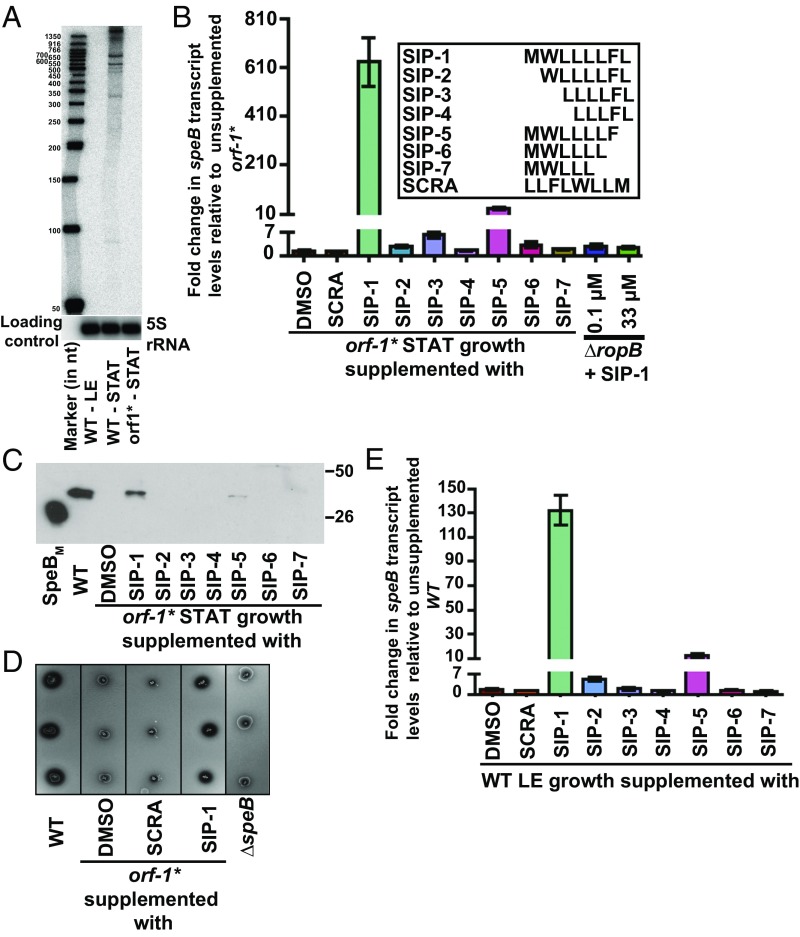
Fig. 2.
Synthetic peptides containing the amino acid sequences of SIP activate speB expression. (A) The orf1 gene, encoding SIP, is expressed during the stationary phase of GAS growth. Total RNA extracted from WT GAS grown to either the late exponential (LE; A600 ∼ 1.0) or stationary (STAT) phase of growth and orf-1* mutant grown to the STAT phase of growth were analyzed by Northern blot. (B, Inset) Amino acid sequences of the synthetic peptides (SIP-1–SIP-7) used in the experiment. (B) SCRA peptide of an identical length and amino acid composition as SIP-1 but differing in the order of sequence was used as a negative control. The orf-1* mutant strain was grown in chemically defined medium (CDM) to the early STAT phase (A600 ∼ 1.7) and supplemented with either 100 nM indicated synthetic peptide or the carrier for the synthetic peptides (DMSO). After 60 min of incubation, transcript levels of speB were assessed by qRT-PCR. The orf-1* mutant strain supplemented with DMSO was used as a reference, and fold changes in speB transcript levels relative to the reference are shown. (C) Western immunoblot analysis of secreted SpeB in filtered growth media from the indicated samples. Cell growth and synthetic peptide supplementation were performed as described in A. Growth media collected were probed with anti-SpeB polyclonal rabbit antibody and detected by chemiluminescence. The masses of molecular weight markers in kilodaltons (kDa) are marked. (D) Milk plate clearing assay to assess the ability of various SIPs to induce SpeB protease activity in the orf-1* mutant. (E) Addition of SIP-1 and SIP-5 decouples the growth phase dependency of speB expression in WT GAS. The WT GAS was grown in CDM to the mid-exponential growth phase (A600 ∼ 0.6), and cells were incubated with 100 nm of each synthetic peptide for 60 min. Transcript levels of speB were assessed by qRT-PCR, and the fold change in speB expression relative to DMSO-supplemented growth is shown.