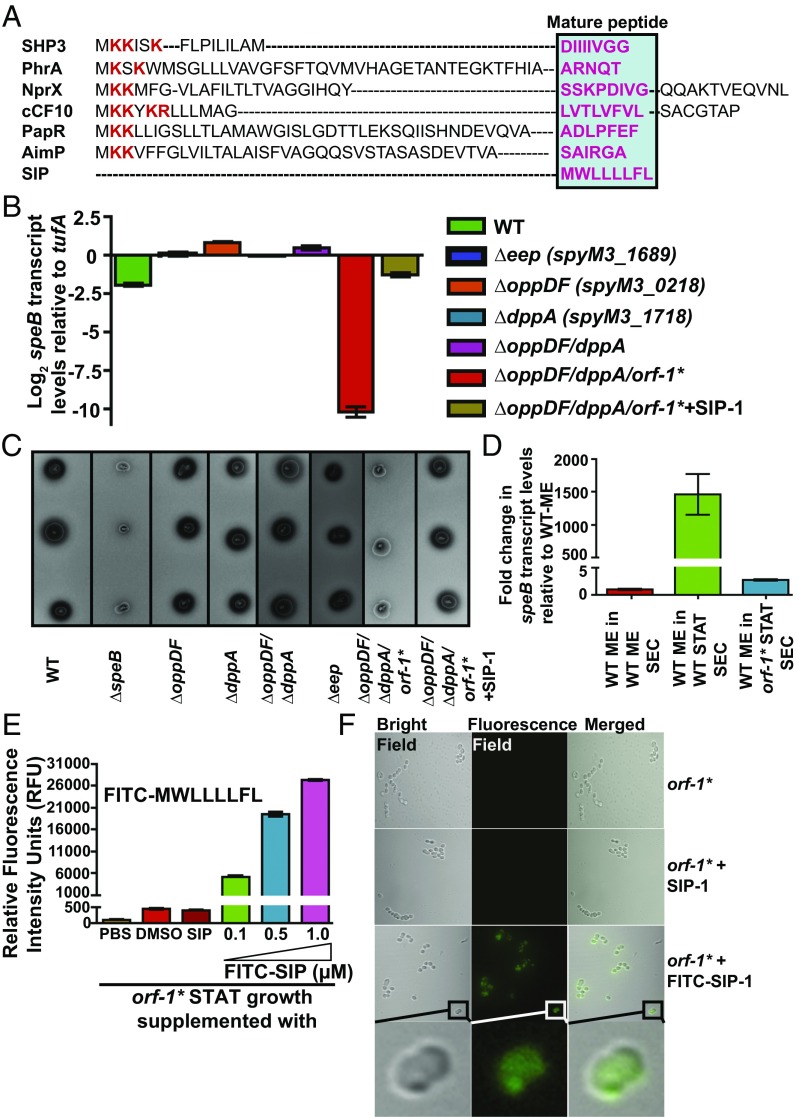
Fig. 3.
Eep protease, Opp, and Dpp do not participate in SIP biosynthesis. (A) Alignment of amino acid sequences of characterized propeptides specific for each founding member of RRNPP family regulators. The propeptides of small hydrophobic peptide 3 (SHP3) from Streptococcus pyogenes, phosphatase regulator A (PhrA) from Bacillus subtilis, peptide signal for neutral protease regulator (NprX) from B. cereus, peptide controlling conjugative transfer of plasmids (cCF10) from E. faecalis, peptide activating PlcR (PapR) from B. cereus, arbitrium communication peptide (AimP) from phage Phi3T, and SIP from S. pyogenes are shown. The positively charged residues characteristic of bacterial peptide signals are shown in red, and the amino acid sequence corresponding to each mature peptide is boxed and highlighted in pink. Transcript levels of the speB (B) and SpeB protease activity of SpeB (C) were assessed in the indicated strains by qRT-PCR and milk plate clearing assay, respectively. (D) Genetic inactivation of sip results in loss of regulatory activity in the secreted component of GAS growth. A qRT-PCR analysis of speB transcript level in WT GAS grown in cell‐free culture supernatants obtained from the indicated strains is shown. Secretome preparation and secretome swap assay were performed as described in SI Appendix, Supplemental Materials and Methods. Triplicate biological replicates were grown on two different occasions and analyzed in duplicate. The data were graphed as the mean ± SD. ME, mid-exponential phase of growth; ME SEC, total secretome prepared from mid‐exponential growth phase; orf-1* STAT SEC, total secretome prepared from the stationary growth phase of the orf-1* mutant; WT STAT SEC, total secretome prepared from the stationary growth phase of WT GAS. (E, Inset) Amino acid sequence of the synthetic peptide SIP-1 with fluorescein modification at its amino terminus (FITC–SIP-1) used in the experiment. (E) The orf-1* mutant strain was grown in chemically defined medium (CDM) to the early stationary phase (STAT, A600 ∼ 1.7) and supplemented with either the indicated synthetic peptide or the carrier for the synthetic peptides (DMSO). Unmodified SIP-1 was added at a final concentration of 1 μM, whereas varying concentrations of FITC–SIP-1 were used. After 60 min of incubation at 37 °C, cells were washed three times with sterile PBS, suspended in PBS, and lysed. Fluorescence measurements were obtained with clarified cell lysates using excitation and emission wavelengths of 480 nm and 520 nm, respectively. The unsupplemented orf-1* mutant strain was used as a reference, and changes in relative fluorescence units (RFU) relative to the reference are shown. (F) Confocal microscopy images of the orf-1* mutant strain either unsupplemented or supplemented with the indicated synthetic peptide. Synthetic peptide addition to the orf-1* mutant strain was performed as described in E. For each sample, bright-field, fluorescence-field, and merged images are shown. (Bottom) Magnified view of the FITC–SIP-1–supplemented growth. [Scale bars: 63.4 μm × 63.4 μm (y axis × x axis) at 100× magnification.]