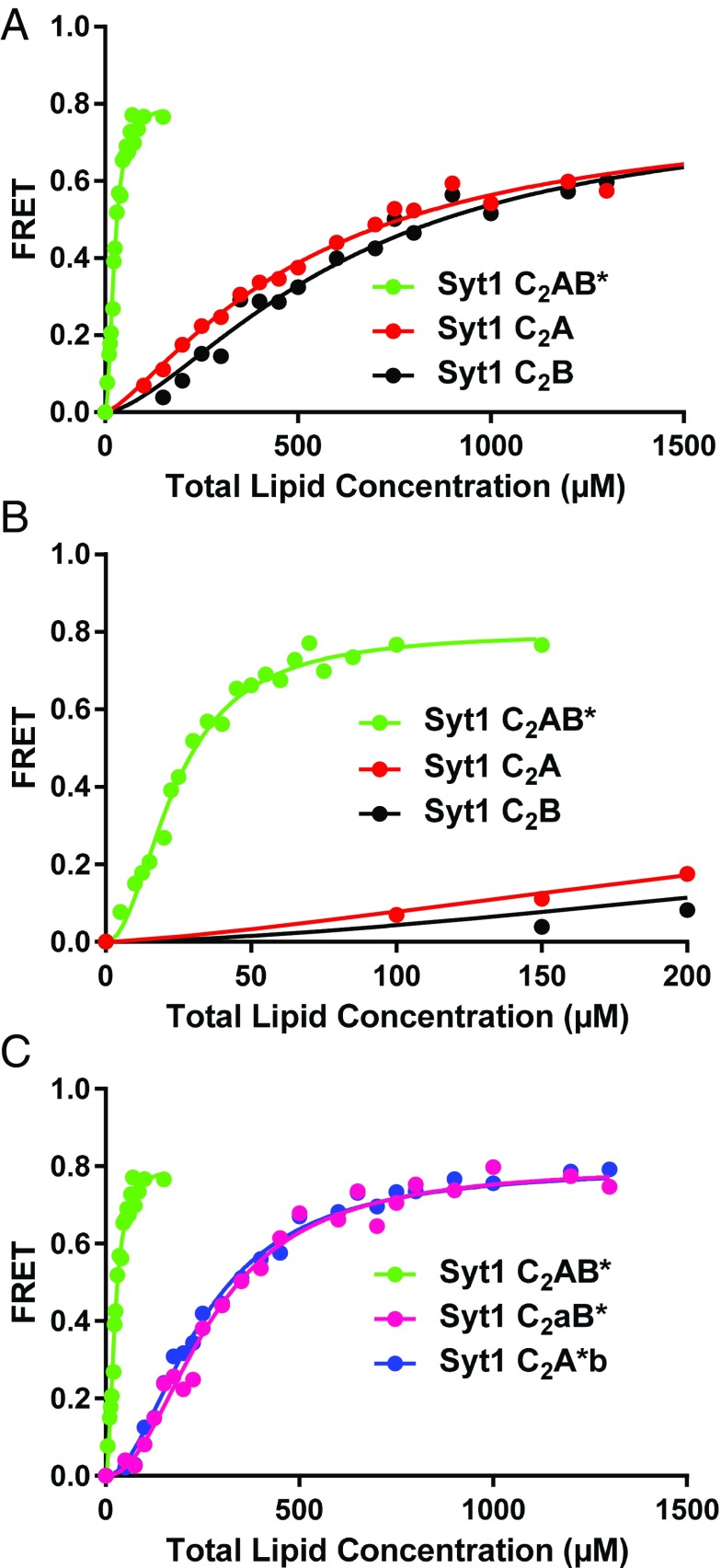
Fig. 5.
The Syt1 C2 domains have similar membrane affinities and cooperate in membrane binding. (A) Titrations of 100 nM Syt1 C2A domain, C2B domain and C2AB* fragment (the * in C2AB* denotes that the fluorescent probe is attached to the C2B domain) with liposomes lacking PIP2 in the presence of 1 mM Ca2+. Binding was monitored from the FRET developed between a donor BODIPY-FL probe attached to the proteins and a rhodamine acceptor present in the liposomes. Each point represents the average of at least three measurements performed with different liposome preparations. (B) The same titrations shown in A but changing the x axis to better show the points of the titrations obtained at low liposome concentrations. (C) Liposome titrations of 100 nM WT Syt1 C2AB* fragment and mutant fragments where the Ca2+-binding sites of the C2A domain (C2aB*) or C2B domain (C2A*b) were disrupted with D178A,D230A,D232A or D309A,D363A,D365A mutations. All of the data were fit to a Hill function.