Abstract
The presence of subcutaneous nodular onchocercosis was investigated at slaughter of 151 red deer (Cervus elaphus) (107 juveniles and 44 adults) between October–December 2015. The prevalence of subcutaneous nodules was 56%. Nodules were located in the lumbar region of the back in 96% of the cases, and 38% of the infected red deer had additional parasitic nodules in other body locations, such as rump, thorax, forelimbs and neck. The number of nodules per deer was 1–10 in two-thirds of the affected animals, and only 2% had more than 50 nodules. Significant differences in prevalence of nodule presence were found between adult (81%) and juvenile red deer less than two years of age (45%) (p = 0.0001). Species confirmation and identification was done on 14 randomly selected nodules taken from the lumbar region of 14 deer of different geographical origin, by sequencing the mitochondrial 12S, 16S and nad5 gene fragments. The sequences were more or less identical (12S = 99–100%; 16S = 93–100%; nad5 = 92–100%) to previously published sequences for Onchocerca flexuosa. The high prevalence of nodular onchocercosis caused by O. flexuosa in red deer in Sweden shows that the investigated areas in the southern third of Sweden have favorable vector (simuliids and ceratopogonids) conditions and suitable environment for the maintenance of the parasite. To our knowledge, this is the first systematic study of O. flexuosa in red deer in Sweden.
Keywords: Onchocerca flexuosa, Cervid, DNA genotyping, Filarioidea, Onchocercidae, Vector-borne helminthes
Graphical abstract

Highlights
-
•
Over 50% of the red deer in the study were infected by nodular onchocercosis.
-
•
Identification of parasite Onchocerca flexuosa by sequencing of mitochondrial fragments 12S, 16S and nad5.
-
•
Prevalence of nodular onchocercosis was significantly higher in adult red deer than juveniles.
-
•
The subcutaneous nodules were especially localized to the lumbar region.
1. Introduction
Hunters and game processing units in Sweden have noted subcutaneous nodules on red deer (Cervus elaphus) carcasses at inspection for decades. The cause and distribution of the nodules within the Swedish red deer population has not been generally known or scientifically investigated. The parasites found in the nodules were hypothesized to belong to the genus Onchocerca (Filarioidea: Onchocercidae). The genus Onchocerca comprises over 30 species with a worldwide distribution mainly associated wild and domestic ungulates (e.g. cervids, horses, bovids, camelids.) (Morales-Hojas et al., 2006, Lefoulon et al., 2017). Two species are found as adult worms in dogs (O. lupi) and humans (O. volvulus) (Wildenburg et al., 1997, Anderson, 2000, Morales-Hojas et al., 2006).
Currently, members of the family Cervidae are hosts of six described species of Onchocerca; O. flexuosa, O. jakutensis (syn. O. tubingensis), O. skrjabini (syn. O. tarsicola), O. garmsi, O. eberhardi and O. cervipedis. In addition, an undescribed species was recently found in North American cervids (McFrederick et al., 2013). In European red deer, four species of Onchocerca are represented (Bain and Schulz-Key, 1974). Adult parasites have defined locations within the subcutaneous tissue in the host (Bain and Schulz-Key, 1974, Morandi et al., 2011, Hidalgo et al., 2015). In red deer, O. flexuosa occurs on the dorsal areas of back and flank (Bain and Schulz-Key, 1974, San-Miguel et al., 2003, Hidalgo et al., 2015), while O. jakutensis can be found on the posterior dorsal region and thighs (Bain and Schulz-Key, 1974) and the upper parts of the extremities (Demiaszkiewicz, 1993). Onchocerca garmsi is present over the sternum (Schulz-Key et al., 1976), while O. skrjabini generally is located in the subcutis over the tibio-tarsal and radio-carpal joints of red deer without causing nodular lesions (Bain and Schulz-Key, 1974, Wildenburg et al., 1997). Two species; O. flexuosa and O. jakutensis, form subcutaneous nodules (Bain and Schulz-Key, 1974) and may look similar in appearance. However, O. flexuosa can be identified by localization and morphology and by DNA sequencing of certain regions in the mitochondrial DNA (Krueger et al., 2007).
Previous reports of O. flexuosa in red deer (Cervus elaphus) are limited to central and southern European countries. Prevalence varies widely between reports and different countries (Hidalgo et al., 2015); from 5% in Czech Republic (Páv and Zajíček, 1981) to 96% in Germany (Schulz-Key, 1975). Other studies of O. flexuosa report prevalence 13% in Romania (Dulceanu and Ghitescu, 1986), 60% in Poland (Demiaszkiewicz, 1998), 33% in Ukraine (Zhelizniak, 2003) and 63% in Belarus (Shimalov and Shimalov, 2003). In Spain, regional differences have been reported; in the central part of the country the prevalence of O. flexuosa was 24% (San-Miguel et al., 2003) and 33% (Santín-Durán et al., 2001), while in the northwest it was 85% (Hidalgo et al., 2015). No previous systematic study of the parasite and its presence in red deer has been done in any of the Nordic countries.
The genus Onchocerca is transmitted as larvae by obligate insect vectors including blackflies (Simuliidae); Simulium ornatum, Prosimulium nigripes and biting midges (Ceratopogonidae) (Frank et al., 1968), in which the parasite undergoes necessary changes to become third stage larvae that are infectious, before it can be transmitted by vectors blood meal on the host (Taylor et al., 2007). The parasite can cause the parasitic disease onchocercosis. Subcutaneous nodules caused by O. flexuosa in red deer are formed as a result of host immune reaction against the parasite (Plenge-Bönig et al., 1995, Wildenburg et al., 1997, Hidalgo et al., 2015). The lesions caused by subcutaneous nodules may lead to condemnation of the carcass at slaughter (Muller, 1979) and can affect the health of the animal but do not usually cause serious illness because of the localization (Plenge-Bönig et al., 1995). However, potential deleterious effects/or negative impacts of O. flexuosa infection on the health and welfare of red deer is unknown (Hidalgo et al., 2015).
The aims of this study were to investigate the geographical presence and anatomical distribution of subcutaneous nodular onchocercosis in Swedish red deer and to identify the parasite species involved by mitochondrial DNA sequence analysis.
2. Materials and methods
2.1. Study area
Our study was carried out in 12 out of 13 counties in the southern third of Sweden covering an area of 126 500 km2. The inland area of this region has a cold climate with snow during the winter and coniferous forest as dominant vegetation while the landscape along the southern coast is characterized by deciduous forest and a warmer climate. Proximity to the North Atlantic Ocean and the Gulf Stream gives Sweden, despite its northern geographic location, mild winters with an average temperature in January of −2 °C. The average annual temperature is 10 °C (average temperature is 18 °C in August) (http://www.smhi.se/kunskapsbanken/klimat/sveriges-klimat-1.6867 [2015.11.19]). Average annual precipitation is 500–800 mm, reaching peak values in the summer and autumn. During the winter the precipitation is mostly snow.
2.2. Sampling procedures
Between 1st October and 30th December 2015, the carcasses of red deer from eight slaughterhouses were examined when skinned by the slaughterhouse staff during regular handling. Occurrence of subcutaneous nodular onchocercosis and animal data (sex, age) was documented in a protocol. There were two age groups: juveniles (<2 years old) and adults (>2 years old) based on weight, body shape, and size of antlers. Infection intensity was grouped by number of nodules per carcass; 0, 1–10, 11–50 and > 50 nodules. The localization of nodules on the skinned body was registered as found on lumbar back, rump, flank, chest, shoulder or forelimbs, or combinations of these areas. Throughout the study, fourteen samples of subcutaneous nodules were taken from the back of red deer from seven deer farms (two deer per farm) in different geographical areas. Genomic DNA from nematodes was extracted manually and preserved in 70% ethanol for molecular analysis.
2.3. Genotyping
Genomic DNA from parasites was extracted with NucleoSpin® Tissue kit. Amplification of two mitochondrial rRNA genes, 12S and 16S, and one mitochondrial protein coding gene NADH dehydrogenase subunit 5 (nad5) was then performed in a PCR 2720 Thermal Cycler (Applied Biosystems) by primer combinations 12S OvC and OvB, 16S OvC and OvB and nd5 OvA and OvC used by Morales-Hojas et al. (2006) and Krueger et al. (2007). PCR reactions of 25 μl consisted of 1 unit AmpliTaq Gold DNA polymerase (Applied Biosystems) with associated 1x Buffer II, 3 mM MgCl2, 200 μM of each dNTP, 0,2 μM of each primer (Forward and Reverse) and 2 μl of extracted DNA. PCR cycling parameters for the DNA reaction included denaturation at 94 °C for 3 min followed by 35 cycles of 94 °C for 45 s, 50 °C for 1 min and 72 °C for 30 s followed by a post-amplification extension at 72 °C for 5 min. To ensure that there was no contamination of the samples, agar gel electrophoresis (1%) with no template controls (NTC) was performed to control that PCR amplicons generated DNA fragments of expected size. The amplicons were sent to Macrogen Inc, Amsterdam for sequencing. Sequence quality was initially analyzed using CLC Main Workbench v5.6.1 (CLC Bio, Aarhus, Denmark). All high quality sequences were then imported to Mesquite v3.04, aligned in MAFFT v7.0 and finally submitted for a nucleotide identity match using the Basic Local Alignment Search Tool (BLAST®) through the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi [2017.07.28]). From this analysis, representative sequences of each gene have been uploaded to European Nucleotide Archive (ENA).
2.4. Data analysis
To investigate the influence of sex and age on presence and number of subcutaneous nodules in individual deer, Chi-square tests were performed as described by Rózsa et al. (2000). Analysis was carried out using Minitab Express™ 1.3.0 (419068).
3. Results
3.1. Presence and distribution of nodules
A total of 151 red deer (104 males and 47 females) from 12 out of 13 counties in the southern part of Sweden were included in the study (Gävleborg, Halland, Jönköping, Kalmar, Skåne, Stockholm, Södermanland, Uppsala, Värmland, Västmanland, Västra Götaland and Östergötland). The majority of animals originated from red deer farms (86%, n = 130). Five were free-ranging hunted deer (3%) and 16 were of unknown status (11%).
Subcutaneous nodules were found in 56% (95% CI 48–64, n = 84 individuals). Due to difficulty of extracting and counting entire parasites from the nodules, prevalence and intensity of infection are reported as nodules per deer. The majority (95%) of infected individuals had less than 50 nodules, 67% of the parasitized deer had 1-10 nodules and 29% had 11-50 nodules (Table 1). Adult red deer had a significantly (p < 0.0001) higher prevalence (82%, n = 36) than juvenile deer up to 2 years of age (45%, n = 48). The prevalence of infection in juveniles and adult animals were 35% (95% CI 24–45) and 96% (95% CI 87–100) for males, as well as 77% (95% CI 61–93) and 67% (95% CI 47–87) for females, respectively. A significant difference in prevalence (p = 0.0072) was observed between males (48%, n = 50) and females (72%, n = 34). In this study, males were overrepresented by juvenile animals (75% of the juveniles were males and only 22% of adults). In contrast, females where more equally distributed across age classes, i.e. 24% and 48% of juveniles and adults, respectively.
Table 1.
Distribution of subcutaneous nodules in red deer among different age groups.
| Age category | Number of nodules |
|||
|---|---|---|---|---|
| 0 | 1–10 | 11–50 | >51 | |
| Juveniles (<2 years) | 59 | 35 | 13 | 0 |
| Adults | 8 | 21 | 11 | 4 |
| Total | 67 | 56 | 24 | 4 |
In the vast majority, 96% (95% CI: 92–100, n = 81) of infected red deer, nodules were localized to the lumbar region. Of these deer, 31% (95% CI: 21–41, n = 25) also had nodules on the rump, flanks and upper forelegs. Furthermore, 7% (95% CI: 2–13, n = 6) had nodules on the chest and front. Only three individuals (4%, 95% CI: 0–8) had nodules in all regions; back, flank, chest, front and forelegs.
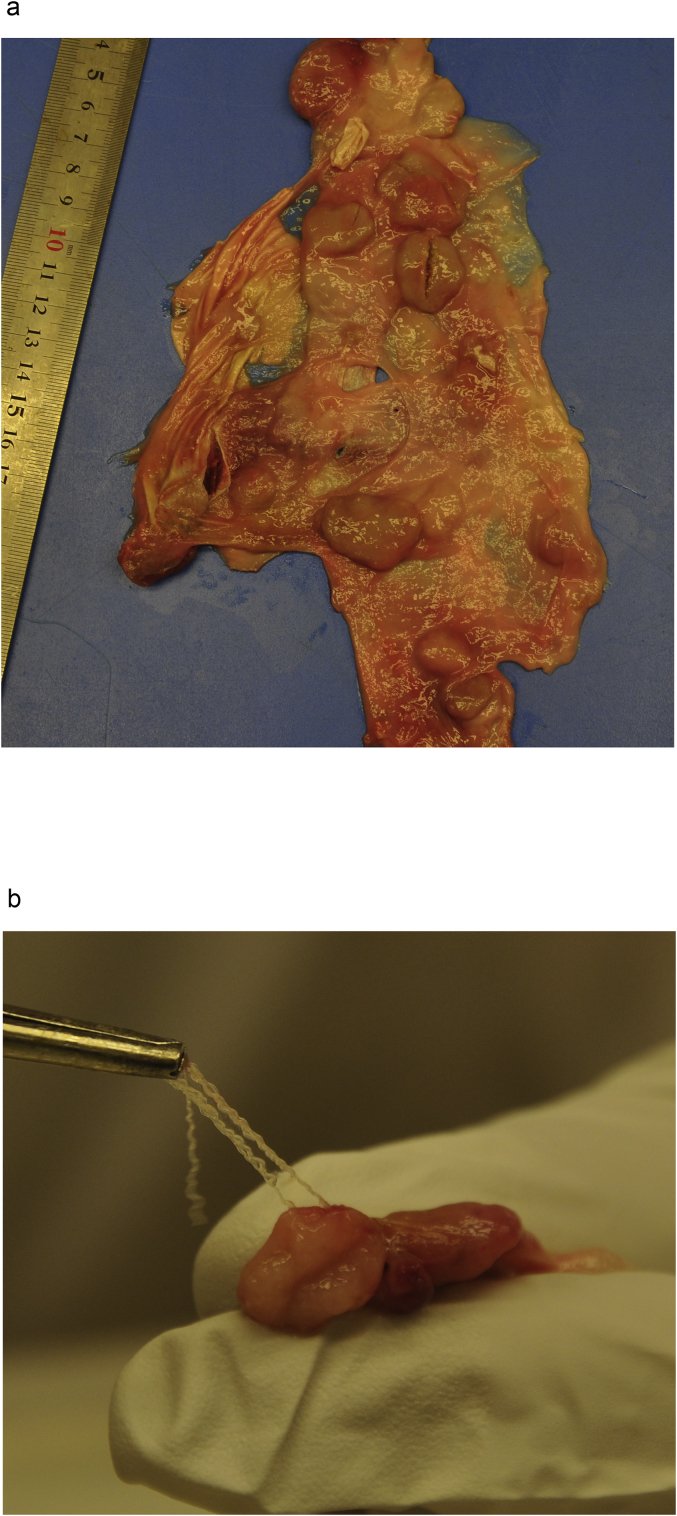
The parasite nodules were rounded, oval to irregular in form and somewhat flattened, with a diameter from 4 mm up to 35 mm (Fig. 1a). Generally, the nodules were firm and typically pale yellow with occasional red coloration due to hemorrhage. It was equally as common to observe solitary nodules or dense clusters of nodules. The threadlike parasites were tightly intertwined within the fibrous nodule but sections of adult worms could be manually extracted with a pair of tweezers (Fig. 1b).
Fig. 1.
Subcutaneous nodules. a) in the subcutaneous fascia from the rump of a carcass b) parts of the threadlike O. flexuosa extracted from a subcutaneous nodule.
3.2. Genotyping and molecular findings
Sequencing the mitochondrial fragments of the rRNA 12S and 16S regions as well as nad5 produced aligned fragments, which after trimming of the primer sites, were between 428 and 477 bp long. The sequences were 477 bp for 12S, 430 bp for 16S and 428 bp for nad5 and with 2, 7 and 8 single nucleotide differences, respectively (http://www.ebi.ac.uk/ena/data/view/LT732682-LT732697[2017.07.28]). According to BLAST searches our sequences were more or less identical (12S = 99–100%; 16S = 93–100%%; nad5 = 92–100%) with previously published sequences for Onchocerca flexuosa. Closest identities (100%) for our most common haplotypes were with GenBank accessions LT732687 (16S); LT732696 (nad5); and LT732682 (12S).
4. Discussion
In this study, over 50% of the examined red deer had subcutaneous nodules induced by Onchocerca flexuosa. The majority (96%) of the infected red deer had subcutaneous nodules on the lumbar region, which is consistent with the preferred location of this parasite (Bain and Schulz-Key, 1974).
In accordance with similar studies (Santín-Durán et al., 2001, San-Miguel et al., 2003, Hidalgo et al., 2015) there is a significant difference in prevalence of nodules between adults (81%) and juvenile deer up to two years of age (45%). The most plausible explanation for the observed difference between the age groups in this study is related to differences in the rate of exposure to the parasite by vectors. The prepatency period of O. flexuosa is at least six months (Schulz-Key, 1975). Young red deer born in late spring could be infected with larvae or young adult parasites that were not yet circumscribed by nodules when the investigation was done in the fall. Infected filarial parasites (including Onchocercids) also tend to be long-lived (Wildenburg et al., 1997). Thus it is more probable that parasite nodule intensity will increase rather than disappear with age of the host individuals.
Unlike other studies (Santín-Durán et al., 2001, Hidalgo et al., 2015) we found a significant difference in infection rate between male (48%) and female (72%) deer in the present study. This is most likely explained by the uneven sex-distribution between and within the different age categories, rather than sex-related biological differences in susceptibility to O. flexuosa. Thus, we believe that it is more relevant to compare infection divergence in relation to age rather than between sexes.
To compare our results of O. flexuosa prevalence with other studies is somewhat difficult because the number of similar studies are few (Hidalgo et al., 2015). European reports on O. flexuosa in red deer are either focused on morphological species identification, prevalence of microfilariae in the skin or sex determination and number of adult parasites inside the nodules. Only a few studies compare the intensity of the number of nodules per individual (Santín-Durán et al., 2001, San-Miguel et al., 2003, Hidalgo et al., 2015).
The strongest red deer population is in the southern half of Sweden, but red deer exist also in fragmented populations in the northern half. Investigations on the prevalence of subcutaneous nodules in the red deer population where it is present throughout the country and during all seasons is needed to get a comprehensive picture of parasite presence in red deer in Sweden. Additionally, it would be interesting to do a systematic investigation and find out if there are differences in prevalence between animals kept in enclosures close to black fly habitat where infection pressure can be high due to permanent presence of intermediate hosts, versus the situation in free-ranging deer.
The presence of suitable vectors in different habitats also likely varies, as vectors are dependent on a wide range of environmental factors for optimal survival. A modification of these environmental factors would thus lead to changes in prevalence of O. flexuosa intermediate host. Today there are probably multiple simulids present throughout Sweden that are living in the same area as both parasite and host and therefor could potentially act as vectors.
Today, the impact of a warmer climate on Onchocerca species and their vectors is largely unknown (Krueger et al., 2007). Nevertheless, this study contributes to the continued identification of Onchocerca species in red deer and their geographical distribution, which is necessary to achieve greater understanding of their evolution and potential impact on animals including humans (Morales-Hojas, 2009).
5. Conclusion
Subcutaneous nodular onchocercosis in Swedish red deer is caused by O. flexuosa and prevalence is significantly higher in adults (>2 years of age) compared to younger animals. High prevalence and intensity of nodular onchocercosis in Swedish red deer indicate favorable vector and environmental conditions for the spread of this parasite in the south-central parts of Sweden.
Funding
This work was supported by the Swedish University of Agricultural Sciences -Department of Biomedical Sciences and Veterinary Public Health, and the Swedish National Veterinary Institute.
Acknowledgements
Thanks to staff members at game-handling establishments, deer farm owners and hunters in Sweden for co-operation and kindness. Special thanks to Moa Skarin, Anders Jarnemo, Jonas Malmsten, Kurt Svensson, Fredrik Eklund and Fredrik Widemo for guidance, expertise and thoughts. Thanks to Behdad Tarbiat and Aleksija Neimanis for graphic and language editing, respectively.
References
- Anderson R.C. second ed. CABI Publishing; Wallingford: 2000. Nematode Parasites of Vertebrates: Their Development and Transmission. [Google Scholar]
- Bain O., Schulz-Key H. Les Onchocerques du Cerf européen: redescription d’O. flexuosa (Wedl, 1856) et description d’O. tubingensis n.sp. et O. tarsicola n.sp. Tropenmed Parasitol. 1974;25:437–449. [PubMed] [Google Scholar]
- Demiaszkiewicz A.W. Prevalence of onchocercinae in deer. Pol. J. Vet. Sci. 1998;7:13–14. [Google Scholar]
- Demiaszkiewicz A.W. Redescription of Onchocerca jakutensis (gubanov, 11964) (nematoda, filarioidea) Acta Parasitol. 1993;38:124–127. [Google Scholar]
- Dulceanu N., Ghitescu F. Incidentá si unele aspecte morfopatologice in oncocercoza subcutana la cerb (Cervus elaphus) Agro. Res. Mol. 1986;19:107–109. [Google Scholar]
- Frank W., Wenki P., Scerb H. Untersuchungen an Onchocerca flexuosa (Nematoda:Filarioidea) eines Parasiten des Rothirsches Cervus elaphus. Verh. Dtsch. Zool. Ges. 1968;3:540–550. [Google Scholar]
- Hidalgo M.R., Martínez A., Carreño R.A., González S., Ferreras M.C., Díez N. Levels of infection, pathology and nodule size of Onchocerca flexuosa (Nematoda: onchocercidae) in red deer (Cervus elaphus) from northern Spain. J. Helminthol. 2015;89:326–334. doi: 10.1017/S0022149X1400011X. [DOI] [PubMed] [Google Scholar]
- Krueger A., Fischer P., Morales-Hojas R. Molecular phylogeny of the filaria genus Onchocerca with special emphasis on Afrotropical human and bovine parasites. Acta Trop. 2007:101–114. doi: 10.1016/j.actatropica.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Lefoulon E., Giannelli Alessio, Makepeace B.L., Mutafchiev Y., Townson S., Uni S., Verocai G.G., Otranto D., Martin C. Whence river blindness? The domestication of mammals and host-parasite co-evolution in the nematode genus Onchocerca. Int. J. Parasitol. 2017 doi: 10.1016/j.ijpara.2016.12.009. [DOI] [PubMed] [Google Scholar]
- McFrederick Q.S., Haselkorn T.S., Verocai G.G., Jaenike J. Cryptic Onchocerca species infecting North American cervids, with implications for the evolutionary history of host associations in Onchocerca. Parasitol. 2013;140:1201–1210. doi: 10.1017/S0031182012001758. [DOI] [PubMed] [Google Scholar]
- Morales-Hojas R. Molecular systematics of filarial parasites, with an emphasis on groups of medical and veterinary importance, and its relevance for epidemiology. Infec. Genet. Evol. 2009;9:748–759. doi: 10.1016/j.meegid.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Morales-Hojas R., Cheke R.A., Post R.J. Molecular systematics of five Onchocerca species (Nematoda: filarioidea) including the human parasite, O. volvulus, suggest sympatric speciation. J. Helminthol. 2006;80:281–290. [PubMed] [Google Scholar]
- Morandi F., Krueger A., Panarese S., Sarli G., Verin R., Nicoloso S., Benazzi C., Galuppi R. First description of nodular onchocercosis (Onchocerca jakutensis) in free-ranging Italian red deer (Cervus elaphus) J. Wildl. Dis. 2011;47:963–967. doi: 10.7589/0090-3558-47.4.963. [DOI] [PubMed] [Google Scholar]
- Muller R. Identification of Onchocerca. In: Taylor A.E.R., Muller R., editors. Identification of Parasites and Their Vectors- 17 Th Symposium of the British Society for Parasitology. Blackwell; Oxford: 1979. pp. 175–206. [Google Scholar]
- Páv J., Zajíček D. Health conditions of ungulates in the region with air pollution. Práce VULHM. 1981;58:213–228. [Google Scholar]
- Plenge-Bönig A., Krömer M., Büttner D.W. Light and electron microscopy studies on Onchocerca jakutensis and O. flexuosa of red deer show different host-parasite interactions. Parasitol. Res. 1995;81:66–73. doi: 10.1007/BF00932419. [DOI] [PubMed] [Google Scholar]
- Rózsa L., Reiczigel J., Majoros G. Quantifying parasites in samples of hosts. J. Parasitol. 2000;86:228–232. doi: 10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- San-Miguel J.M., Álvarez G., Rodríguez-Vigal C., Luzón M. Nodular onchocercosis of red deer in central Spain. Vet. Parasitol. 2003;114:75–79. doi: 10.1016/s0304-4017(03)00097-9. [DOI] [PubMed] [Google Scholar]
- Santín-Durán M., Alunda J.M., de la Fuente C., Hoberg E.P. Onchocercosis in red deer (Cervus elaphus) from Spain. J. Parasitol. 2001;87:1213–1215. doi: 10.1645/0022-3395(2001)087[1213:OIRDCE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Schulz-Key H. Untersuchungen über die Filarien der Cerviden in Süddeutschland. 2. Die Filarien des Rothirsches (Cervus elaphus) Tropenmed Parasitol. 1975;26:348–358. [PubMed] [Google Scholar]
- Schulz-Key H., Bain O., Wenk P. Untersuchungen über die Filarien der Cerviden in Süddeutschland. 4. Onchocerca garmsi Bain und Schulz-Key, 1976, eine subkutane Filarie des Rothirsches (Cervus elaphus) Tropenmed Parasitol. 1976;27:229–232. [PubMed] [Google Scholar]
- Shimalov V.V., Shimalov V.T. Helminth fauna of cervids in belorussian polesie. Parasitol. Res. 2003;89:75–76. doi: 10.1007/s00436-002-0700-x. [DOI] [PubMed] [Google Scholar]
- Taylor M.A., Coop R.L., Wall R.L. third ed. Blackwell publishing Ltd; Victoria Australia: 2007. Veterinary Parasitology. [Google Scholar]
- Wildenburg G., Plenge-Bönig A., Renz A., Fischer P., Büttner D.W. Distribution of mast cells and their correlation with inflammatory cells around Onchocerca gutturosa, O. tarsicola, O. ochengi, and O. flexuosa. Parasitol. Res. 1997;83:109–120. doi: 10.1007/s004360050220. [DOI] [PubMed] [Google Scholar]
- Zhelizniak P. New species filaria (Spirurida Onchocercidae) in fauna of Ukraine. Vestn. Zool. 2003;37:73–75. [Google Scholar]