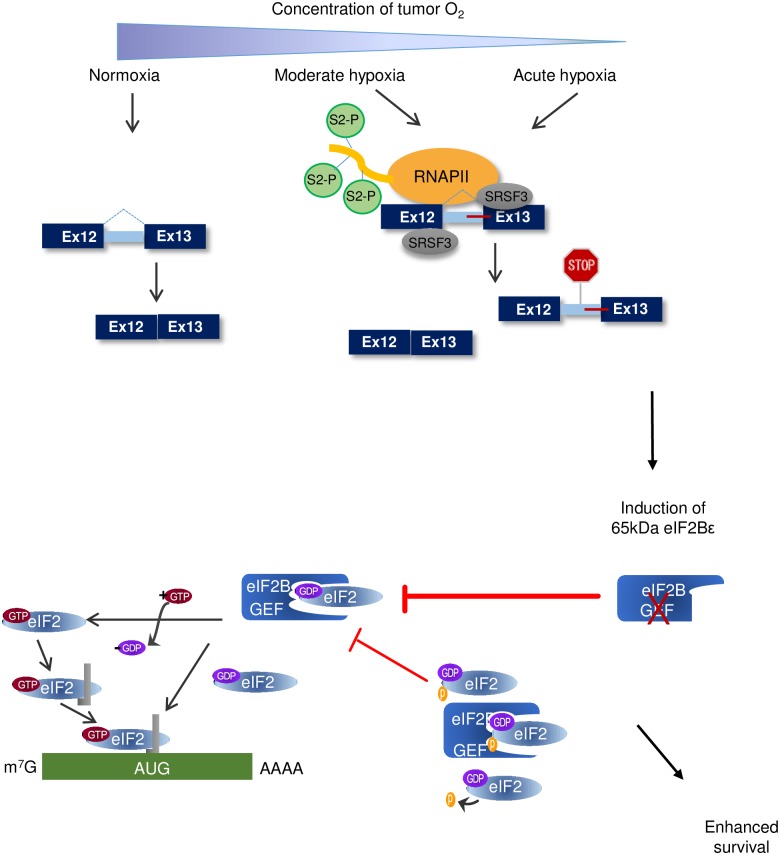
Fig 8. Model of hypoxia-induced intron retention in EIF2B5 as a mechanism to reduce translation and enhance survival in head and neck cancer cells during periods of prolonged or acute hypoxia.
Upper: Under acute or prolonged hypoxia, increased phosphorylation of Ser2-RNAPII accumulates specifically at EIF2B5 intron 12. Binding of SRSF3 is increased under hypoxia at this locus, which contains a weak splice site and alternate downstream splice site. Altogether, oxygen deprivation leads to an accumulation of intron12-containing EIF2B5 transcripts, which results in a truncated reading frame due to insertion of a premature termination codon (PTC). Lower: Retention of intron 12 under hypoxia results in a 65kDa isoform of eIF2Bε. This isoform lacks the functional guanine exchange factor (GEF) domain and acts opposite to the full-length isoform to inhibit translation during periods of prolonged hypoxia, which ultimately confers a survival advantage to SQ20B cells under hypoxia.