Figure 1. Mechanisms of interplay between aging and cancer.
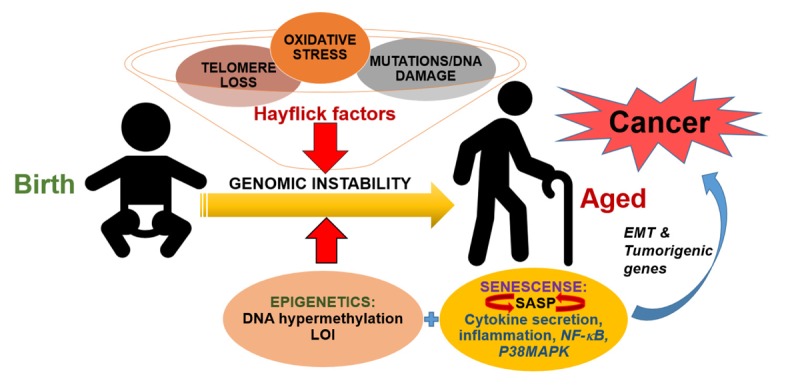
During normal aging, accumulation of damaged DNA, telomere loss, mutations and subsequent oxidative stress-dependent changes, the so-called “Hayflick factors” lead to overall genomic instability. Meanwhile, the increasing abundance of aberrant epigenetic changes such as DNA hypermethylation and loss of imprinting (LOI) can also significantly contribute to genomic instability. Yet another complication arises as these advanced senescent cells develop the senescence-associated secretory phenotype (SASP), which is accompanied by robust cytokines secretion that can act on premalignant cells to promote a highly inflammatory tissue microenvironment. SASP itself is initiated by certain pro-inflammatory cytokines which serve as potent activators of important inflammatory signals, including the nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinase (p38MAPK) pathways. These pathways, once activated, can also establish a feed-forward loop via the transcription of cytokine target genes that in turn activate SASP. Overall, this results in activation of an epithelial-to-mesenchyme transition (EMT) and tumorigenic phenotype in cells, due to increased transcription of gene products related to these processes. This ultimately leads to cancer initiation and progression.
