Figure 7. PAO1-induced neutrophil trans-epithelial migration using a murine in-vitro Transwell co-culture model requires cPLA2α signaling from the neutrophil.
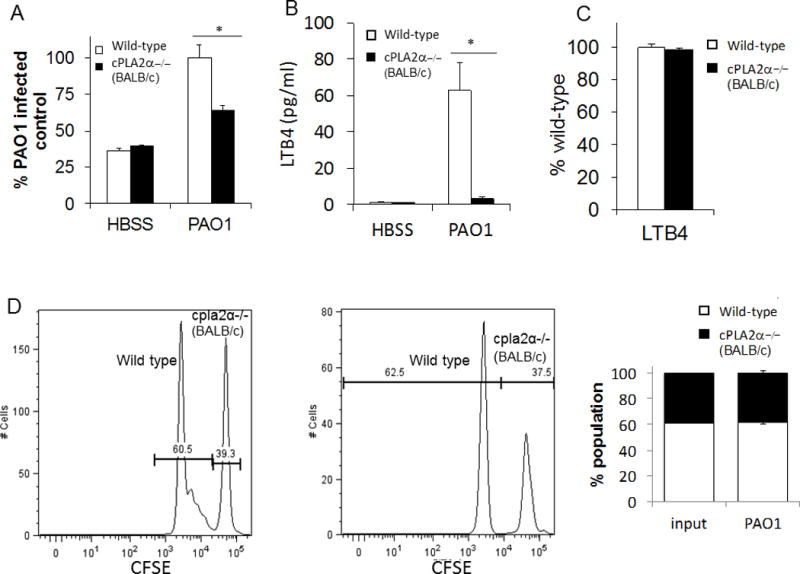
A) Total bone marrow was isolated from cPLA2α deficient mice on a BALB/c background and littermate controls then supplied to the basolateral compartment of MLE12 monolayers. Neutrophils were allowed to migrate across an MLE monolayer infected with PAO1 or mock-infection (HBSS). Relative neutrophil migration was assessed by myeloperoxidase activity in the apical compartment following 2h migration. Total myeloperoxidase activity was corrected for using standard curves generated for each bone marrow source. Data are shown as mean corrected value +/− standard deviation. B) Following migration, the apical compartment was sampled for the presence of LTB4 by ELISA. C) Neutrophils from cPLA2α deficient mice and littermate controls migrated across MLE12 monolayers towards an imposed gradient of LTB4 (5ng/ml). Total myeloperoxidase activity was corrected for using standard curves generated for each bone marrow source and data are shown as mean corrected value +/− standard deviation. D) Bone marrow from WT and cPLA2α −/− mice were differentially labeled with CFSE and allowed to undergo a combined migration in a near 1:1 ratio. The ratio of neutrophils collected from the apical compartment was assessed after two hours of migration across a PAO1-infected epithelium by flow cytometry. Representative histograms, gated on Ly6G+ neutrophils, and average proportion detected in multiple tests are shown. Experiments were performed on at least three occasions with n≥3 technical replicates. P values were calculated by paired Student’s t-test. P values ≤0.05 was consider significant.
